Treatment outcomes of locally advanced hypopharyngeal squamous cell carcinoma
Introduction
Hypopharyngeal squamous cell carcinoma (HPSCC) is a common head and neck cancer in Taiwan (1). The disease has a locally aggressive pattern and the worst prognosis among all head and neck subsites (2,3). Smoking and alcohol intake are the main risk factors for these patients. Common symptoms include dysphagia and odynophagia. Because of nonspecific early symptoms, 70–85% of patients with HPSCC are usually diagnosed at stage III or IV stage (4). The survival outcome is poor despite improved diagnostic technique and developments in surgical and non-surgical treatment in the recent decades. Radical surgery with or without adjuvant radiotherapy or concurrent chemoradiotherapy (CCRT) is traditionally the main initial treatment modality for advanced HPSCC. Due to high morbidity after the surgical treatment, non-surgical treatment was widely studied for voice preservation and swallowing function, thus better quality of life. Some studies reported that organ preservation treatment resulted in comparable outcomes to primary surgery (5-7). However, there is still no consensus for the optimal treatment of advanced HPSCC from previous results (5-8). Previous laryngeal preservation studies were mostly based on patients with laryngeal cancer or mixed subsites and stages of head and neck cancer. Therefore, the aim of our study was to report the treatment outcomes with comparative analysis between primary surgery and primary CCRT for patients with advanced HPSCC.
Methods
Between September 2002 and September 2013, 367 patients with advanced-stage (III, IVA and IVB) HPSCC were reviewed. All patients received pretreatment evaluation and computed tomography (CT) or magnetic resonance imaging (MRI) of the head and neck region for staging prior primary treatment. Patients with distant metastasis at diagnosis (n=1), previous cancer history (n=20), no treatment (n=33), treatment with laser surgery (n=1), treatment with chemotherapy alone (n=10), treatment with radiotherapy alone (n=3), synchronous malignancy (n=30) and incomplete CCRT (n=12) were excluded, leaving 257 patients for analysis.
One hundred and thirty-three patients were treated with primary surgery (SX group). Among the SX group, three patients received induction chemotherapy and 83 patients with adverse prognostic factors such as positive surgical margins, extracapsular extension of lymph nodes, metastasis in more than 2 lymph nodes also received adjuvant radiotherapy (n=19) or concurrent chemoradiotherapy (n=64). The remaining 50 patients underwent surgery alone (Figure 1). Types of surgery included extended laryngopharyngectomy, partial laryngopharyngectomy, total laryngectomy, and total laryngectomy with partial pharyngectomy. Bilateral or unilateral neck lymph node dissection was undertaken at the discretion of the primary surgeon based on clinical diagnosis or their clinical judgment. Patients receiving laryngomicrosurgery or biopsy alone were excluded.
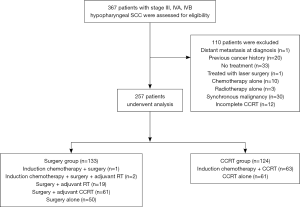
Among the 124 patients treated with definitive concurrent chemoradiotherapy (CCRT group), 63 patients received induction chemotherapy followed by CCRT and 61 patients underwent definitive CCRT alone (Figure 1). After the CCRT treatment, 8 patients underwent salvage neck dissection, 9 patients underwent salvage laryngopharyngectomy and 16 patients underwent both salvage laryngopharyngectomy and neck dissection for residual disease. The concurrent chemotherapy regimen was at the discretion of the medical oncologist and mostly included a cisplatin-based regimen (weekly 30–40 mg/m2/wk). Induction chemotherapy regimen consisted of FP (5-fluorouracil, cisplatin) or TPF (5-fluorouracil, taxotere, cisplatin). For patients treated with radiotherapy, the initial RT treatment field was to irradiate the entire tumor and regional lymphatics with adequate margins with 6MV X-ray beams via 2D, 3D or IMRT (which was the major technique after 2004). The prescribed dose was 1.8–2 Gy per fraction per day, given 5 days per week. The dose for initial RT fields ranged from 46 Gy to 50.4 Gy. The boost RT volume included primary tumor or tumor bed and positive lymph node regions. In the adjuvant setting, most patients received a total RT dose of 60.8–66 Gy. In the CCRT group, most patients received a total RT dose of 66–72 Gy unless intolerable. For those receiving induction chemotherapy, the treatment response was evaluated by RECIST criteria (9) and mostly defined by CT scans. After the primary treatment, patients were followed in the outpatient department and image studies were routinely arranged to detect recurrence. A CT scan or MRI of the head and neck was performed 2 to 3 months after RT and then annually for 5 years, or as clinically indicated after primary treatment. Bone scans and abdominal sonography were arranged when clinically indicated.
The primary endpoints of this study were overall survival rate (OS) and disease-free survival (DFS), and the secondary endpoints were loco-regional recurrence rate (LRR) and distant metastasis rate (DMR). The duration of survival was calculated from the time of pathological diagnosis to the date of event or to the most recent follow-up date. Survival rates were estimated by Kaplan-Meier method and survival curves were compared by log-rank test in univariate analysis. The Cox-regression model was used for multivariate analysis. A P-value of less than 0.05 was used to indicate statistical significance.
The study was approved by the ethnic committee of our hospital. The IRB number for this study is 104-4642B. The SPSS ver.22.0 statistical software was used for data processing (IBM Corp., Armonk, NY, USA).
Results
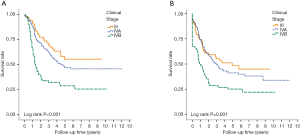
A total of 257 patients met the inclusion criteria and the characteristics of all patients treated were summarized in Table 1. The mean age was 53.8 (range, 33–85 years). Most patients were male (97.3%), smokers (83.3%) and alcohol drinkers (78.6%). All patients were staged using the TNM classification system (7th AJCC edition) (10). Fifty-four patients (21%) had stage III tumors, 133 patients (51.8%) had stage IVA tumors and 70 patients (27.2%) had stage IVB tumors. There was no significant difference between two the groups in age, sex, performance status, proportion of smokers and alcohol drinkers.
Table 1
| Characteristics | All patients (n=257) | Surgery group (n=133) | CCRT group (n=124) | P value |
|---|---|---|---|---|
| Age, mean (SD) | 53.8 (10.4) | 53.8 (9.6) | 53.7 (11.1) | 0.12 |
| Male sex | 250 (97.3) | 130 (97.7) | 120 (96.8) | 0.71 |
| Stage | <0.01 | |||
| III | 55 (21.4) | 36 (27.1) | 19 (15.3) | |
| IVA | 132 (51.4) | 82 (61.7) | 50 (40.3) | |
| IVB | 70 (27.2) | 15 (11.3) | 55 (44.4) | |
| T classification | <0.01 | |||
| T1 | 15 (5.8) | 6 (4.5) | 9 (7.3) | |
| T2 | 36 (14.0) | 20 (15.0) | 16 (12.9) | |
| T3 | 77 (28.8) | 52 (39.1) | 25 (20.2) | |
| T4 | 129 (51.4) | 55 (41.4) | 74 (59.7) | |
| N classification | <0.01 | |||
| N0 | 55 (21.4) | 29 (21.8) | 26 (21) | |
| N1 | 44 (17.1) | 25 (18.8) | 19 (15.3) | |
| N2 | 135 (52.5) | 77 (57.9) | 58 (46.8) | |
| N3 | 23 (8.9) | 2 (1.5) | 21 (16.9) | |
| Histology grade | <0.01 | |||
| WD or MD | 124 (48.2) | 104 (78.2) | 20 (16.1) | |
| PD or UD | 57 (22.2) | 27 (20.3) | 30 (24.2) | |
| Unclassified | 76 (29.6) | 2 (1.5) | 74 (59.7) | |
| Performance status (ECOG) | 0.78 | |||
| 0–1 | 218 (84.8) | 112 (84.2) | 106 (85.5) | |
| 2–3 | 39 (15.2) | 21 (15.8) | 18 (14.5) | |
| Tobacco smokers | 214 (83.3) | 111 (83.5) | 103 (83.1) | 0.99 |
| Alcohol use | 202 (78.6) | 110 (82.7) | 92 (74.2) | 0.10 |
Data are shown as number (%). CCRT, concurrent chemo-radiotherapy; WD, well differentiation; MD, moderately differentiation; PD, poorly differentiation; UD, un-differentiation.
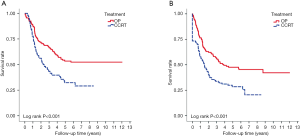
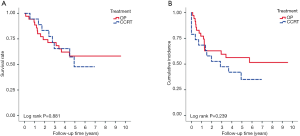
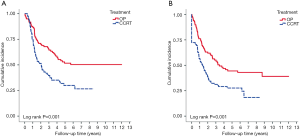
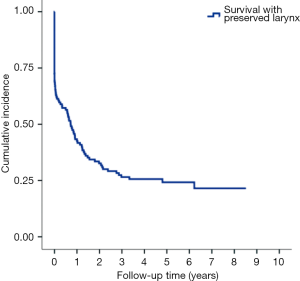
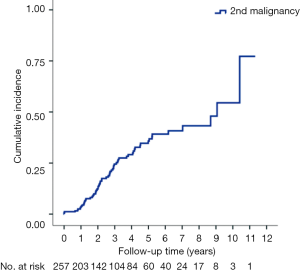
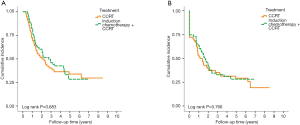
With median follow up of 5.83 years (range, 0.05 to 12.05 years) for surviving patients, the 5-year OS, DFS, LRR and DMR of all patients were 44%, 39%, 60% and 23%, respectively. Clinical stage was a significant predictor for prognosis as shown in Figure 2. In the univariate analysis (Table 2), age <65 (vs. ≥65) years, sex and subsite showed no significant correlation with OS, DFS, LRR and DMR. There were 60 deaths (45%) in the SX group and 77 deaths (62%) in the CCRT group; 44 recurrences (33%) in the SX group and 65 recurrences (52%) in the CCRT group. In the CCRT group, 34 (27.4%) patients had persistent disease, 26 (21%) patients had locoregional recurrence, 22 (17.7%) had distant metastasis after treatment. In the SX group, 34 (25.6%) patients had locoregional recurrence, 21 (15.8%) had distant metastasis. The earlier clinical stage (III vs. IV) showed significant correlation with improved OS (54.3% vs. 40.9%, P=0.030) and a trend of improved DFS (47.5% vs. 35.9%, P=0.129). The treatment groups (OP vs. CCRT) showed significant correlation with improved OS (53.4% vs. 32.3%, P<0.001), DFS (47.6% vs. 28.4%, P<0.001) and LRR (28.2% vs. 52.6%, P<0.001), but not with DMR (21.2% vs. 24.9%, P=0.276) (Figures 3,4). In subgroup analysis, the treatment groups (OP vs. CCRT) showed significant correlation with improved OS (51.7% vs. 29.9%, P=0.001) and DFS (44.5% vs. 27.6%, P=0.001) in clinical stage IVA–B disease, but no significant correlation with OS (58.2% vs. 47.7%, P=0.881) and DFS (56.2% vs. 35.1%, P=0.239) in stage III disease (Figures 5,6). Further multivariate analysis (see Table 3) based on different covariates showed that clinical stage and treatment group were independent predictors of OS. Earlier stage predicted improved OS (P=0.03) and decreased DMR (P=0.03). Furthermore, treatment with primary CCRT appeared to increase risk of death by 45% and risk of loco-regional recurrence by over 50% and was a poor predictor for both OS (P=0.008), DFS (P=0.002) and LRR (P=0.000). In the CCRT group, the addition of induction chemotherapy (Upfront chemotherapy plus CCRT vs. definitive CCRT) had no significant correlation with OS (28.4% vs. 33.9%, P=0.683) and DFS (28.1% vs. 29.2%, P=0.766) (Figure 7) and 38% of patients had partial response at least after completion of induction chemotherapy. Survival with preserved larynx was defined as survival without local disease evolution, tracheotomy or tracheostoma. The 5-year rate of survival with preserved larynx was 24.2% (Figure 8). And the 5-year cumulative incidence of secondary malignancy was 64% (Figure 9).
Table 2
| Variable | No. of patients (%) | OS | DFS | LRR | DMR | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-yr OS, % | P | 5-yr DFS, % | P | 5-yr LRR, % | P | 5-yr DM, % | P | |||||
| Age (yr) | 0.852 | 0.763 | 0.548 | 0.712 | ||||||||
| <65 | 216 (84%) | 43.9 | 38.9 | 39.4 | 22.7 | |||||||
| ≥65 | 41 (16%) | 42.8 | 36.7 | 43.1 | 26.4 | |||||||
| Sex | 0.460 | 0.534 | 0.548 | 0.625 | ||||||||
| Male | 250 (97%) | 43.7 | 38.4 | 40.1 | 23.4 | |||||||
| Female | 7 (3%) | 45.7 | 42.9 | 33.3 | 16.7 | |||||||
| Subsites | 0.188 | 0.294 | 0.777 | 0.386 | ||||||||
| PS | 220 (86%) | 45.8 | 40.1 | 39.3 | 22.5 | |||||||
| PC/PW | 37 (14%) | 32.1 | 28.9 | 43.9 | 27.8 | |||||||
| Stage | 0.030 | 0.129 | 0.422 | 0.031 | ||||||||
| III | 55 (21.4%) | 54.3 | 47.5 | 33.4 | 12.5 | |||||||
| IV | 202 (78.6%) | 40.9 | 35.9 | 42.0 | 26.5 | |||||||
| Treatment | 0.000 | 0.000 | 0.000 | 0.276 | ||||||||
| OP | 133 (52%) | 53.4 | 47.6 | 28.2 | 21.2 | |||||||
| CCRT | 124 (48%) | 32.3 | 28.4 | 52.6 | 24.9 | |||||||
OS, overall survival; DFS, disease-free survival; LRR, loco-regional recurrence; DM, distant metastasis; PS, pyriform sinus; PC, post-cricoid; PW, posterior pharyngeal wall; OP, operation; CCRT, concurrent chemo-radiotherapy.
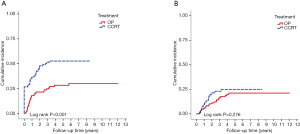
Table 3
| Variable | Overall survival | Disease-free survival | Locoregional recurrence rate | Distant-metastasis rate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||||
| Age (ref: <65 |
1.04 (0.65–1.65) | 0.87 | 1.07 (0.69–1.65) | 0.77 | 1.12 (0.64–1.95) | 0.70 | 1.24 (0.57–2.69) | 0.59 | |||
| ECOG (ref: 0–1 |
0.93 (0.57–1.49) | 0.75 | 0.87 (0.55–1.35) | 0.53 | 0.85 (0.47–1.53) | 0.59 | 0.5 (0.18–1.41) | 0.19 | |||
| Differentiation (ref: WD or MD |
1.45 (0.92–2.28) | 0.11 | 1.47 (0.96–2.25) | 0.08 | 1.44 (0.83–2.51) | 0.20 | 1.29 (0.60–2.75) | 0.52 | |||
| Clinical stage (ref: III |
1.66 (1.05–2.63) | 0.03 | 1.32 (0.88–2.01) | 0.18 | 1.10 (0.65–1.87) | 0.72 | 2.88 (1.12–7.40) | 0.03 | |||
| Subsites (ref: PS |
1.44 (0.92–2.26) | 0.11 | 1.36 (0.88–2.11) | 0.17 | 1.12 (0.63–2.01) | 0.70 | 1.56 (0.72–3.41) | 0.26 | |||
| Treatment (ref: CCRT |
0.55 (0.35–0.86) | 0.008 | 0.52 (0.34–0.79) | 0.002 | 0.37 (0.21–0.63) | 0.000 | 0.50 (0.24–1.04) | 0.06 | |||
HR, hazard ratio; ref, reference; WD, well differentiation; MD, moderately differentiation; PD, poorly differentiation; UD, un-differentiation; PS, pyriform sinus; PC, post-cricoid; PW, posterior pharyngeal wall; CCRT, concurrent chemo-radiotherapy; OP, operation.
Discussion
Hypopharyngeal squamous cell carcinoma has the worst prognosis among all head and neck cancers (11). Patients with hypopharyngeal squamous cell carcinoma are often diagnosed when at advanced stages (12). The regime for these patients involves achieving a balance of cancer treatment, decreased treatment-related morbidity, preservation of organ function and a satisfactory quality of life. In the past, combination of primary surgery alone or in combination with post-operative radiotherapy was the standard treatment. However, due to the poor prognosis and poor quality of life after surgery (13), several studies started to investigate induction chemotherapy in 1980s (14). Lefebvre et al. (15) reported a result from phase III study conducted by the European Organization for Research and Treatment of Cancer randomly treating 194 patients with stage II to IV HPSCC with either surgery and postoperative RT or induction chemotherapy, followed by radiotherapy for patients with complete response and surgery for non-responding patients. The 5-year survival was 35% and 30% respectively without any significant difference between these two groups. Another retrospective study published by Lajtman and Menestar also showed no significant difference in 5-year OS between non-surgical and surgical therapy (16). From these trials, the larynx-conserving approach appeared to be an alternative treatment and upfront surgery is no longer the only standard treatment for hypopharyngeal cancer. Furthermore, concomitant chemoradiotherapy was widely accepted as organ preservation treatment in recent decades (17,18). A randomized trial conducted by the Radiation Therapy Oncology Group and the Head and Neck Intergroup (RTOG 91-11) reported that radiotherapy plus concurrent cisplatin is superior to sequential therapy or radiotherapy alone for achieving locoregional control and laryngeal preservation in patients with locally advanced resectable laryngeal cancer (19). On the basis of these results, concurrent chemoradiotherapy has been regarded as the gold standard treatment for HPSCC in term of organ preservation.
However, Kuo et al. (20) analyzed 3,968 hypopharyngeal cancer patients from Surveillance, Epidemiology, and End Results database. Kuo found that surgery with radiation still has better survival rates than radiation alone with hazard ratio of 1.71. Another study, reviewing the laryngeal cancer patients from the US national cancer database (21) also showed decreased survival in past decades was related to a significant increase in the use of CCRT and decrease in aggressive surgery. Currently, the best treatment to achieve a balance between organ preservation and aggressive surgery is still unknown. Thus, in the present study, we compared the outcome between primary surgery with or without adjuvant RT/CCRT and definitive CCRT in patients with stage III–IV HPSCC. The five-year overall survival (44%) was comparable to the results of previous reports (3,8,22,23). The 5-year OS, DFS and LRR were significantly improved with primary surgery over primary CCRT in both univariate and multivariate analysis. A retrospective study presented by Axon et al. (24) echoed our findings. Tsou et al. (25) retrospectively analyzed 202 patients with HPSCC and found a significantly better survival rate in the surgery-first group than the CCRT-first group for stage III to IV disease. The decrease of tumor burden achieved by surgery may be associated with improvement of loco-regional recurrence and play an important role for advanced HPSCC. In the present study, the cumulative incidence of 5-year loco-regional recurrence was statistically significant lower in primary surgery group (28.2%) than primary CCRT group (52.6%). There was no significant difference for the 5-year distant metastasis rate between the primary surgery group (21.2%) and the primary CCRT group (24.9%). These results may be explained by a decrease of locoregional recurrence that benefits survival in the primary surgery group.
The major difference between our study and the EORTC 24891 prospective trial is that the majority of our patients had stage IV disease (78.6%) in comparison to only 37% of patients with stage IV disease in the EORTC trial (23). In the present study, we also demonstrated that there was no significant difference between primary surgery and primary CCRT in term of OS (58.2% vs. 47.7%, P=0.881) and DFS (56.2% vs. 35.1%, P=0.239) in stage III disease. Patients with stage III disease could therefore be potential candidates for organ preservation treatment. A larger prospective randomized trial is needed in order to clarify this.
Our study had some limitations. Due to the retrospective nature, the patients’ characteristics at pre-treatment clinical stage were not balanced between surgical and non-surgical groups. There were more patients with T4 or N3 stage in the CCRT group than in the surgery group. Besides, the lack of information about treatment side effects or functional outcomes added to the review's limits. In addition, the follow-up time was not long enough and long-term follow-up is needed to confirm our findings.
For advanced HPSCC, pretreatment tumor volume may play an important role as a prognostic factor. William et al. (26) analyzed 404 patients with squamous cell carcinoma of the head and neck treated with definitive RT and found that patients with higher disease stage (T3–4) tend to have larger tumors and tumor volume may be a useful predictor of outcome. A similar finding resulted from retrospective analysis conducted by Anna et al. of review of 78 patients who underwent definitive CCRT for stage III–IV squamous cell carcinoma of the hypopharynx, oropharynx and larynx. And this study showed that primary tumor volume was a significant prognostic factor better than T or N stage (27). In our study, patients with advanced T stage (≥T3) were balanced in both groups, however, patients with higher T stage may present with higher pretreatment tumor volume and could be more favorable candidates for undergoing primary surgery than primary CCRT to achieve better local control and survival outcome.
Conclusions
Hypopharyngeal cancer is a distinct clinical entity of head and neck cancer with a relatively poor prognosis. In the present study, we demonstrated that treatment with primary surgery for patients with advanced stage HPSCC (stage III, IVA and IVB) is correlated with significant better overall, DFS and loco-regional control rates than primary CCRT. However, due to the limited sample size, further large prospective studies are still needed to identify patients with advanced hypopharyngeal cancer suitable for primary CCRT or organ preservation treatment.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tro.2018.03.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethnic committee of our hospital. The IRB number for this study is 104-4642B. Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Taiwan Ministry of Health and Welfare (2016) Taiwan Ministry of Health and Welfare Ministry of Health and Welfare, Taipei City, Taiwan. Cancer registry annual report of Taiwan 2012. 2016.
- Hall SF, Groome PA, Irish J, et al. The natural history of patients with squamous cell carcinoma of the hypopharynx. Laryngoscope 2008;118:1362-71. [Crossref] [PubMed]
- Newman JR, Connolly TM, Illing EA, et al. Survival trends in hypopharyngeal cancer: a population-based review. Laryngoscope 2015;125:624-9. [Crossref] [PubMed]
- Buckley JG, MacLennan K. Cervical node metastases in laryngeal and hypopharyngeal cancer: a prospective analysis of prevalence and distribution. Head Neck 2000;22:380-5. [Crossref] [PubMed]
- Chang MF, Wang HM, Kang CJ, et al. Treatment results for hypopharyngeal cancer by different treatment strategies and its secondary primary--an experience in Taiwan. Radiat Oncol 2010;5:91. [Crossref] [PubMed]
- Kim JW, Kim MS, Kim SH, et al. Definitive chemoradiotherapy versus surgery followed by adjuvant radiotherapy in resectable stage III/IV Hypopharyngeal Cancer. Cancer Res Treat 2016;48:45-53. [Crossref] [PubMed]
- Urba SG, Moon J, Giri PG, et al. Organ preservation for advanced resectable cancer of the base of tongue and hypopharynx: a Southwest Oncology Group Trial. J Clin Oncol 2005;23:88-95. [Crossref] [PubMed]
- Harris BN, Biron VL, Donald P, et al. Primary surgery vs chemoradiation treatment of advanced-stage hypopharyngeal squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg 2015;141:636-40. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Edge S BD, Compton CC. AJCC cancer staging manual, 7th ed. New York: Springer. 2010.
- Carvalho AL, Nishimoto IN, Califano JA, et al. Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database. Int J Cancer 2005;114:806-16. [Crossref] [PubMed]
- Beauvillain C, Mahe M, Bourdin S, et al. Final results of a randomized trial comparing chemotherapy plus radiotherapy with chemotherapy plus surgery plus radiotherapy in locally advanced resectable hypopharyngeal carcinomas. Laryngoscope 1997;107:648-53. [Crossref] [PubMed]
- Pingree TF, Davis RK, Reichman O, et al. Treatment of hypopharyngeal carcinoma: a 10-year review of 1,362 cases. Laryngoscope 1987;97:901-4. [Crossref] [PubMed]
- Dimery IW, Hong WK. Overview of combined modality therapies for head and neck cancer. J Natl Cancer Inst 1993;85:95-111. [Crossref] [PubMed]
- Lefebvre JL, Chevalier D, Luboinski B, et al. Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst 1996;88:890-9. [Crossref] [PubMed]
- Lajtman Z, Manestar D. A comparison of surgery and radiotherapy in the management of advanced pyriform fossa carcinoma. Clin Otolaryngol Allied Sci 2001;26:59-61. [Crossref] [PubMed]
- Pignon JP, le Maitre A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009;92:4-14. [Crossref] [PubMed]
- Prades JM, Lallemant B, Garrel R, et al. Randomized phase III trial comparing induction chemotherapy followed by radiotherapy to concomitant chemoradiotherapy for laryngeal preservation in T3M0 pyriform sinus carcinoma. Acta Otolaryngol 2010;130:150-5. [Crossref] [PubMed]
- Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 2003;349:2091-8. [Crossref] [PubMed]
- Kuo P, Chen MM, Decker RH, et al. Hypopharyngeal cancer incidence, treatment, and survival: temporal trends in the United States. Laryngoscope 2014;124:2064-9. [Crossref] [PubMed]
- Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope 2006;116:1-13. [Crossref] [PubMed]
- Kim S, Wu HG, Heo DS, et al. Advanced hypopharyngeal carcinoma treatment results according to treatment modalities. Head Neck 2001;23:713-7. [Crossref] [PubMed]
- Lefebvre JL, Andry G, Chevalier D, et al. Laryngeal preservation with induction chemotherapy for hypopharyngeal squamous cell carcinoma: 10-year results of EORTC trial 24891. Ann Oncol 2012;23:2708-14. [Crossref] [PubMed]
- Axon PR, Woolford TJ, Hargreaves SP, et al. A comparison of surgery and radiotherapy in the management of post-cricoid carcinoma. Clin Otolaryngol Allied Sci 1997;22:370-4. [Crossref] [PubMed]
- Tsou YA, Lin MH, Hua CH, et al. Survival outcome by early chemoradiation therapy salvage or early surgical salvage for the treatment of hypopharyngeal cancer. Otolaryngol Head Neck Surg 2007;137:711-6. [Crossref] [PubMed]
- Mendenhall WM, Morris CG, Amdur RJ, et al. Parameters that predict local control after definitive radiotherapy for squamous cell carcinoma of the head and neck. Head Neck 2003;25:535-42. [Crossref] [PubMed]
- Strongin A, Yovino S, Taylor R, et al. Primary tumor volume is an important predictor of clinical outcomes among patients with locally advanced squamous cell cancer of the head and neck treated with definitive chemoradiotherapy. Int J Radiat Oncol Biol Phys 2012;82:1823-30. [Crossref] [PubMed]
Cite this article as: Chen YY, Tsai YT, Tsai MS, Hsu CM, Lai CH, Lu CH, Chen PT, Fang FM, Chen MF, Chen WC. Treatment outcomes of locally advanced hypopharyngeal squamous cell carcinoma. Ther Radiol Oncol 2018;2:17.