The prognostic value of two different radiotherapy techniques in esophageal cancer patients treated with neoadjuvant chemoradiotherapy
Highlight box
Key findings
• Our National Health Insurance Research Database analysis found no significant difference in overall survival between stage II and III esophageal cancer patients receiving neoadjuvant chemoradiotherapy (neo-CRT) followed by surgery, regardless of intensity-modulated radiotherapy (IMRT) or rotational IMRT (rIMRT) treatment. Notably, sex, age, clinical N (cN) category, radiation dose, and hospital volume emerged as crucial independent prognostic factors.
What is known and what is new?
• In Taiwan, the increasing adoption of IMRT, encompassing advanced modalities like IMRT/volumetric-modulated arc therapy and helical tomotherapy (rIMRT), is observed. While IMRT and image-guided radiation therapy demonstrate improved dosimetric performance, their impact on survival rates remains uncertain. Significantly, no discernible survival disparity was observed between IMRT and rIMRT cohorts.
What is the implication, and what should change now?
• Our study underscores the pivotal roles of sex, age, cN category, radiation dose, and hospital volume in predicting survival for esophageal cancer patients undergoing neo-CRT followed by surgery. Importantly, the study affirms no substantial survival distinction between IMRT and rIMRT cohorts. Future research should delve into additional determinants of survival in this patient population.
Introduction
Background
Esophageal cancer stands as the ninth most prevalent cancer in Taiwan, primarily characterized by squamous cell carcinoma (SCC), which accounts for more than 90% of diagnosed cases (1). The aggressive nature of esophageal SCC, combined with its high incidence, highlights the urgent need for effective therapeutic strategies.
Rationale and knowledge gap
Despite advancements in the understanding and management of esophageal cancer, several challenges persist. Aggressive tumor biology, advanced disease stages, and suboptimal responses to therapy have been identified as contributors to poor survival outcomes (2). While treatment guidelines, such as those established by the National Comprehensive Cancer Network (NCCN), provide valuable recommendations, the complexity of treatment decision-making is influenced by various factors, including patient performance status, available hospital resources, physician preferences, and disease-specific variables (3). In this context, a critical knowledge gap exists in understanding the impact of evolving radiotherapy techniques on survival outcomes, particularly in the Asian population with stage II or III esophageal cancer undergoing neoadjuvant chemoradiotherapy (neo-CRT) followed by surgery.
Objective
The overarching objective of this study is to address the identified knowledge gap by systematically analyzing prognostic factors for overall survival (OS) in Asian patients with stage II or III esophageal cancer who have undergone neo-CRT followed by surgery. Specifically, the study aims to evaluate the influence of different radiotherapy techniques on survival rates, with a particular focus on rotational intensity-modulated radiotherapy (rIMRT).
In the landscape of esophageal cancer treatment, radiotherapy plays a pivotal role, especially in the context of neo-CRT. Current recommendations advocate for a modest dose range of 4,000–5,000 cGy for patients undergoing neo-CRT (4,5). Despite the growing adoption of IMRT and its modern extensions, such as rIMRT, in Taiwan, uncertainties persist regarding their impact on survival rates. Neo-CRT enables tumor downstaging, increases resectability, and affect local control and survival (5). Advanced technologies like helical tomotherapy, referred to collectively as rIMRT, have shown promise in achieving favorable dosimetric performance, as reported by Chen et al. (6). Notably, tomotherapy demonstrated sharper dose gradients, improved conformal coverage, enhanced uniformity, and significant reductions in lung (V20) and heart (V30, V45) exposure.
This study aims to fill the existing knowledge gap by comprehensively examining the impact of various radiotherapy techniques on survival outcomes in Asian patients with esophageal cancer. We present this article in accordance with the STROBE reporting checklist (available at https://tro.amegroups.com/article/view/10.21037/tro-23-17/rc).
Methods
Database
This retrospective cohort study collected linked the datasets from Taiwan’s National Health Insurance Research Database (NHIRD), and the Taiwan Cancer Registry (TCR) and Death Registry Records. The NHIRD data are based on Taiwan’s National Health Insurance program; this program has a coverage rate of >99%, rendering NHIRD data representative of empirical data in medical and health research (7). The study findings can serve as a reference for medical and health policymaking and an essential research resource. Since 1997, the Central Medical Insurance Administration—now restructured into the National Health Insurance Administration—has entrusted the National Institutes of Health to promote the establishment of the NHIRD. After 2 years of preparation and consolidation, hospitals in Taiwan began offering academic health insurance in the year 2000. The database has value-added services to facilitate related research.
The TCR was initiated in 1979 and is updated annually by the Ministry of Health and Welfare, Taiwan. The registry initially recorded 20 clinical-pathological variables for cancer, and the number has increased from 20 to 114 during the years from 2002 to 2011. The data are standardized under the government’s entrustment to ensure data accuracy and are reviewed through regular medical record investigations. The diagnosis of malignancy is confirmed through histology. The TCR has excellent data completeness (97%) and quality (8). All study individuals have a hashed and unique personal identification number to link the data between these nationwide databases. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Human Research Ethics Committee of the Institutional Review Board of Chung Shan Medical University Hospital (CS2-20036). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Study population
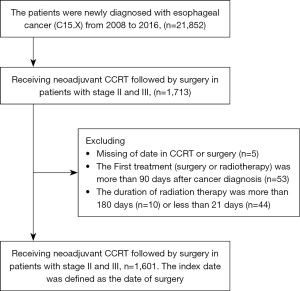
In the TCR dataset, we identified the patients who were diagnosed with esophageal cancer (International Classification of Diseases for Oncology codes: C15.0, C15.1, C15.2, C15.3, C15.4, C15.5, C15.8, and C15.9) at clinical stage II or III (determined according to the AJCC Cancer Staging Manual, Seventh Edition) between 2008 and 2016 and receiving concurrent CRT followed by surgery for treatment. A total of 1,713 patients were captured.
The exclusions criteria included patients with missing data regarding the date of CRT or surgery (n=5), received the first treatment (surgery or radiotherapy) more than 90 days after cancer diagnosis (n=53), and received radiotherapy for more than 180 days (n=10) or less than 21 days (n=44). On the basis of the aforementioned criteria, this study included 1,601 esophageal cancer patients for analysis (Figure 1).
Since there were only five cases with missing data for CCRT or operation, we excluded them from the analysis. We confirmed that their exclusion did not have an impact on the statistical results. For the data included in the analysis, the date of surgery was set as the index date, and followed them until death or ended on December 31, 2017.
Variables
The TCR dataset used for the study included the following variables for analysis: age at diagnosis, sex, diagnosis year, radiotherapy technique, clinical T (cT), clinical N (cN), clinical stage, differentiation, cancer site, radiotherapy dose, body mass index (BMI), smoking status, and hospital volume. Hospital volume was classified based on the average number of people treated for esophageal cancer annually: if the number was among the top 25% in Taiwan, the hospital volume was considered high; if the number was in the top 25–50%, the hospital volume was medium; and if the number was in the bottom 50%, the hospital volume was low. The Death Registry Records were used to verify the survival status and date of death of the patients included in the study.
Statistical analysis
We used the Kaplan-Meier estimator to calculate the 5-year OS rates among different groups, and the log-rank test was used to test the survival difference for the prognostic variable. We referred to Table S1, where the univariate analysis revealed imbalanced baseline characteristics with an absolute standardized difference (ASD) >0.1. These imbalances encompassed various factors, including the year of diagnosis, age at diagnosis, differentiation, cT stage, cN stage, BMI, smoking, hospital volume, and image-guided radiation therapy (IGRT), between IMRT and rIMRT groups prior to propensity score matching (PSM). To mitigate this confounding bias, we meticulously incorporated these covariates into a subsequent multivariable analysis. This comprehensive approach aimed to estimate the adjusted hazard ratio (aHR) of rIMRT in relation to the risk of all-cause mortality. By doing so, we aimed to ensure the appropriate control of potential confounding factors, thereby strengthening the validity of the results obtained from multivariable Cox proportional hazards regression analyses presented in Table 1. The univariate and multivariable Cox proportional hazards regression was used to estimate the HR and 95% confidence interval (CI) of prognostic factors on the risk of all-cause mortality.
Table 1
| Parameters | aHR (95% CI) | P value |
|---|---|---|
| RT type 1 | ||
| Non-IGRT | Reference | |
| IGRT | 1.03 (0.88–1.20) | 0.715 |
| RT type 2 | ||
| IMRT | Reference | |
| rIMRT | 0.90 (0.77–1.05) | 0.053 |
| Year of diagnosis | ||
| 2010–2012 | Reference | |
| 2013–2016 | 0.87 (0.75–1.02) | 0.082 |
| Sex | ||
| Male | 1.80 (1.25–2.59) | 0.002 |
| Female | Reference | |
| Age at diagnosis (years) | ||
| <40 | 1.24 (0.81–1.91) | 0.327 |
| 40–49 | Reference | |
| 50–59 | 0.95 (0.80–1.13) | 0.585 |
| 60–69 | 1.14 (0.94–1.39) | 0.179 |
| ≥70 | 1.69 (1.28–2.24) | 0.001 |
| Cancer site | ||
| Upper third or cervical area | 1.07 (0.87–1.30) | 0.527 |
| Middle third or thoracic | Reference | |
| Lower third or abdominal | 0.97 (0.82–1.15) | 0.731 |
| Origin intermediate or NOS | 1.12 (0.92–1.36) | 0.267 |
| Differentiation | ||
| Missing | 0.98 (0.84–1.14) | 0.815 |
| Well differentiation | 0.68 (0.41–1.12) | 0.126 |
| Moderate differentiation | Reference | |
| Poor differentiation | 1.01 (0.83–1.22) | 0.938 |
| cT stage | ||
| 1 | 0.61 (0.32–1.19) | 0.146 |
| 2 | Reference | |
| 3 | 1.02 (0.82–1.28) | 0.836 |
| 4 | 1.34 (0.99–1.79) | 0.051 |
| cN stage | ||
| 0 | 0.78 (0.62–0.99) | 0.044 |
| 1 | Reference | |
| 2 | 1.03 (0.88–1.20) | 0.729 |
| 3 | 1.21 (0.97–1.51) | 0.100 |
| RT highest dose | ||
| 3,000–<4,000 | 1.14 (0.86–1.52) | 0.366 |
| 4,000–<5,000 | Reference | |
| 5,000–5,040 | 0.93 (0.79–1.09) | 0.354 |
| >5,040 | 1.39 (1.14–1.70) | 0.001 |
| BMI (kg/m2) | ||
| Missing | 1.20 (0.75–1.93) | 0.456 |
| <18.5 | 1.25 (0.99–1.56) | 0.052 |
| 18.5–24 | Reference | |
| >24 | 0.91 (0.76–1.09) | 0.303 |
| Smoking | ||
| Missing | 0.83 (0.49–1.42) | 0.504 |
| Never smoking | Reference | |
| Current smoking | 0.94 (0.73–1.22) | 0.655 |
| Quit smoking | 0.93 (0.71–1.23) | 0.606 |
| Hospital volume | ||
| High | Reference | |
| Medium | 0.99 (0.84–1.17) | 0.887 |
| Low and very low | 1.35 (1.05–1.74) | 0.020 |
aHR was estimated by multiple Cox regression that included the co-variates of year of diagnosis, sex, age at diagnosis, cancer site, histological type, cancer differentiation, cT and cN stage, BMI, smoking, hospital volume for care, IGRT, and RT technique. aHR, adjusted hazard ratio; CI, confidence interval; RT, radiotherapy; IGRT, image-guided radiation therapy; IMRT, intensity-modulated radiotherapy; rIMRT, rotational IMRT; NOS, non-specific; cT, clinical T; cN, clinical N; BMI, body mass index.
We observed the rIMRT/IMRT might be associated with the OS rate in these patients. However, the treatment was not randomly assigned in the observational study, the potential confounding bias should be adjusted by the appropriate method. The PSM was performed to reduce the confounding bias after balance the measured characteristics between the study groups (9). The propensity score was estimated as the probability of the treatment of rIMRT by using logistic regression, and the covariates included year of diagnosis, sex, age at diagnosis, cancer site, histological type, cancer differentiation, cT and cN stage, BMI, smoking, hospital volume for care, IGRT. We used the PSMATCH procedure in SAS software, the algorithm of greedy nearest neighbor matching, and non-replacement paired within 0.01 caliper widths. Finally, there were 305 pairs of PSM IMRT patients and rIMRT patients were selected for analysis. We used the ASD (10) to compare the baseline covariates between groups in this large-sample observational study. The characteristics were balanced when the ASD was <0.1.
All statistical analyses were conducted by using SAS version 9.4 (SAS Institute, Cary, NC, USA). The significance level of 0.05 was used for hypothesis test.
Results
Patient characteristics
Table 2 indicated the characteristics of the patients (n=1,601). Among the patients, 748 (46.7%) patients had cancer located in the middle third of the esophagus or chest, and 1,528 (95.4%) patients were SCC. In addition, 1,333 (83.3%) patients had clinical stage III cancer, 1,243 (77.6%) patients had stage T3 cancer, and 692 (43.2%) patients had stage N1 cancer. High-volume hospitals treated 1,105 (69.0%) patients. Among the radiotherapy techniques, IMRT with IGRT was used in 197 (12.3%) patients, rIMRT with IGRT in 199 (12.4%) patients, IMRT without IGRT in 917 (57.3%) patients, and rIMRT without IGRT in 288 (18.0%) patients. The median follow-up time was 18 months in this study.
Table 2
| Characteristics | Total (n=1,601), n (%) |
|---|---|
| Year of diagnosis | |
| 2010–2012 | 548 (34.2) |
| 2013–2016 | 1,053 (65.8) |
| Sex | |
| Male | 1,509 (94.3) |
| Female | 92 (5.7) |
| Age at diagnosis (years) | |
| <40 | 32 (2.0) |
| 40–49 | 364 (22.7) |
| 50–59 | 719 (44.9) |
| 60–69 | 385 (24.0) |
| 70 | 101 (6.3) |
| Cancer site | |
| Upper third or cervical area | 232 (14.5) |
| Middle third or thoracic | 748 (46.7) |
| Lower third or abdominal | 382 (23.9) |
| Origin intermediate or NOS | 239 (14.9) |
| Histological type | |
| SCC | 1,528 (95.4) |
| Others | 73 (4.6) |
| Differentiation | |
| Missing | 531 (33.2) |
| Well | 36 (2.2) |
| Moderate | 777 (48.5) |
| Poorly | 257 (16.1) |
| Clinical stage | |
| II | 268 (16.7) |
| III | 1,333 (83.3) |
| cT stage | |
| 1 | 25 (1.6) |
| 2 | 192 (12.0) |
| 3 | 1,243 (77.6) |
| 4 | 141 (8.8) |
| cN stage | |
| 0 | 180 (11.2) |
| 1 | 692 (43.2) |
| 2 | 562 (35.1) |
| 3 | 167 (10.4) |
| BMI (kg/m2) | |
| Missing | 348 (21.7) |
| <18.5 | 184 (11.5) |
| 18.5–24 | 723 (45.2) |
| >24 | 346 (21.6) |
| Smoking | |
| Missing | 325 (20.3) |
| Never smoking | 155 (9.7) |
| Current smoking | 808 (50.5) |
| Smoking cessation | 313 (19.6) |
| Hospital volume† | |
| High | 1,105 (69.0) |
| Medium | 372 (23.2) |
| Low and very low | 124 (7.7) |
| IGRT | |
| Non-IGRT | 1,205 (75.3) |
| IGRT | 396 (24.7) |
| RT technique (IMRT or rIMRT) | |
| IMRT | 1,114 (69.6) |
| rIMRT | 487 (30.4) |
†, the hospital volume is based on the average annual number of patients treated for esophageal cancer from 2010 to 2016. If the number is among the top 25% in Taiwan, the hospital volume is high; if the number is in the top 25–50%, the hospital volume is medium; and if the hospital volume is in the bottom 50%, the hospital volume is low. CCRT, concurrent chemoradiotherapy; NOS, non-specific; SCC, squamous cell carcinoma; cT, clinical T; cN, clinical N; IGRT, image-guided radiation therapy; RT, radiotherapy; IMRT, intensity-modulated radiotherapy; rIMRT, rotational IMRT.
The mortality risk and 5-year OS rate
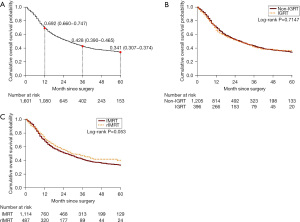
Table 3 presented the all-cause mortality rate was 21.99% (95% CI: 20.58–23.47%) per 1,000 person-months in all study patients. When stratified by IGRT, the all-cause mortality rate was 21.45% (95% CI: 19.86–23.12%) and 23.89% (95% CI: 20.79–27.33%) in the non-IGRT and IGRT cohort, respectively. When stratified by IMRT, the all-cause mortality rate was 22.28% (95% CI: 20.62–24.04%) and 21.17% (95% CI: 18.50–24.12%) in the IMRT and rIMRT cohort, respectively. Figure 2A illustrated the 5-year cumulative OS probability was 0.341 (95% CI: 0.31–0.37) after surgery. Figure 2B showed the IGRT stratified 5-year OS probability was 0.340 (95% CI: 0.31–0.37) and 0.366 (95% CI: 0.31–0.43) in non-IGRT cohort and IGRT cohort, respectively, and the IGRT stratified 5-year OS probabilities was not significant difference (log-rank P=0.715). Figure 2C showed the IMRT stratified 5-year OS probability was 0.328 (95% CI: 0.29–0.36) and 0.400 (95% CI: 0.34–0.46) in IMRT cohort and rIMRT cohort, respectively, and the IMRT stratified 5-year OS probabilities was the borderline statistical significance (log-rank P=0.053).
Table 3
| Parameters | N | Observed person-months | Death, n | All-cause mortality rate (95% CI), % | aHR (95% CI) |
|---|---|---|---|---|---|
| Before PSM | |||||
| All patients | 1,601 | 40,526 | 891 | 21.99 (20.58–23.47) | |
| Non-IGRT | 1,205 | 31,612 | 678 | 21.45 (19.86–23.12) | Reference |
| IGRT | 396 | 8,914 | 213 | 23.89 (20.79–27.33) | 1.03 (0.88–1.21) |
| IMRT | 1,114 | 29,852 | 665 | 22.28 (20.62–24.04) | Reference |
| rIMRT | 487 | 10,674 | 226 | 21.17 (18.50–24.12) | 0.90 (0.77–1.05) |
| After PSM for rIMRT | |||||
| IMRT | 305 | 7,258 | 152 | 20.94 (17.75–24.55) | Reference |
| rIMRT | 305 | 6,463 | 140 | 21.66 (18.22–25.56) | 1.00 (0.78–1.27) |
aHR, the co-variates including the variables of year of diagnosis, sex, age at diagnosis, cancer site, histological type, cancer differentiation, cT and cN stage, BMI, smoking, hospital volume for care, IGRT, and RT technique. All-cause mortality rate, per 1,000 person-months was estimated by the online tool on the website: https://www.openepi.com/PersonTime2/PersonTime2.htm. RT, radiotherapy; CI, confidence interval; aHR, adjusted hazard ratio; PSM, propensity score matching; IGRT, image-guided radiation therapy; IMRT, intensity-modulated radiotherapy; rIMRT, rotational IMRT; cT, clinical T; cN, clinical N; BMI, body mass index.
The aHRs for postoperative OS were showed in Table 3. The aHR was 1.03 (95% CI: 0.88–1.21), which is not statistically significant, in patients with IGRT techniques compared with the non-IGRT cohort. The non-significant aHR of 0.90 (95% CI: 0.77–1.05) was observed between IMRT patients and rIMRT patients. Table 1 showed a multivariate analysis using Cox regression to determine the prognostic factors for OS. Our results indicated that male sex was associated with a poorer OS compared to female sex (aHR =1.80; 95% CI: 1.25–2.59; P=0.002). Additionally, the >70-year-old age group had poorer OS with an aHR of 1.69 (95% CI: 1.28–2.24; P=0.001) when compared to the 40–49-year-old age group. Radiation doses greater than 5,040 cGy were also associated with poorer OS (aHR =1.39; 95% CI: 1.14–1.70; P=0.001) compared to doses within the 4,000 to 5,000 cGy range. High-volume hospitals were associated with a higher OS than low-volume hospitals (aHR =1.35; 95% CI: 1.05–1.74; P=0.020). Finally, patients with cN0 disease had a better prognosis than those with cN1 disease, with an aHR of 0.78 (95% CI: 0.62–0.99; P=0.044). In summary, we identified sex, age, cN category, radiation dose, and hospital volume as independent prognostic factors for OS.
The PSM IMRT cohort and rIMRT cohort
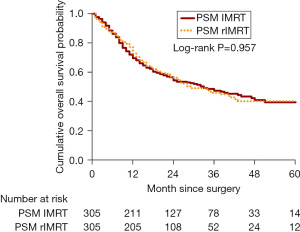
To reduce the effect of confounding bias, we conducted PSM to balance the observed baseline characteristics between IMRT cohort and rIMRT cohort. Before PSM, the unbalanced baseline characteristics, that defined as ASD >0.10, including year of cancer diagnosis, age at diagnosis, cancer histological type, cancer differentiation, BMI, smoking, hospital volume, and IGRT technique, were observed between IMRT cohort and rIMRT cohort (Table S1). After PSM, all baseline characteristics were balanced between two cohorts, however, there were only 305 paired patients selected for analysis (Table S1). After PSM, the 5-year post-surgery OS probability was 0.395 and 0.402 in IMRT cohort and rIMRT cohort, respectively, and the OS probabilities was not significantly different, the log-rank P=0.957 (Figure 3). Table 3 also showed the aHR was 1.00 (95% CI: 0.78–1.27), which is not statistically significant between IMRT and rIMRT after PSM.
In Table 4, we observed the aHR of all-cause mortality between IMRT and rIMRT cohort stratified by baseline characteristics. In the patients with BMI >24 kg/m2, the rIMRT cohort had higher risk of mortality, aHR =2.09 (95% CI: 1.21–3.63), compared with IMRT cohort.
Table 4
| Parameters | aHR (95% CI) of mortality rIMRT compared with IMRT |
|---|---|
| All | 1.00 (0.78–1.27) |
| Stratified by year of diagnosis | |
| 2010–2012 | 0.86 (0.52–1.41) |
| 2013–2016 | 1.03 (0.80–1.32) |
| Stratified by sex | |
| Male | 1.03 (0.82–1.28) |
| Female | 1.02 (0.41–2.54) |
| Stratified by age at diagnosis | |
| Age <50 years | 0.90 (0.56–1.43) |
| Age 50–59 years | 1.06 (0.74–1.53) |
| Age ≥60 years | 1.10 (0.73–1.65) |
| Stratified by clinical stage | |
| Clinical stage II | 0.88 (0.48–1.62) |
| Clinical stag III | 1.07 (0.85–1.36) |
| Stratified by BMI | |
| BMI <18.5 kg/m2 | 0.95 (0.43–2.08) |
| BMI 18.5–24 kg/m2 | 0.96 (0.70–1.32) |
| BMI >24 kg/m2 | 2.09 (1.21–3.63) |
| Stratified by smoking | |
| Never | 0.71 (0.26–1.91) |
| Ever | 0.99 (0.76–1.28) |
| Hospital volume | |
| High | 1.05 (0.81–1.35) |
| Non-high | 0.82 (0.52–1.29) |
| Stratified by IGRT | |
| Non-IGRT | 1.03 (0.79–1.36) |
| IGRT | 1.13 (0.73–1.76) |
rIMRT, rotational IMRT; IMRT, intensity-modulated radiotherapy; aHR, adjusted hazard ratio; CI, confidence interval; BMI, body mass index; IGRT, image-guided radiation therapy.
Discussion
Several factors have been shown to affect the prognosis of esophageal cancer (11-13). Tustumi et al. found that poor differentiation histology and tumor size were associated with worse oncology stages in patients with SCC, but not in those with adenocarcinoma (14). They also found that weight loss and changes in BMI (kg/m2) were predictors of a worse stage at diagnosis in SCC, but not in adenocarcinoma. Similarly, Eloubeidi et al. reported that older age, black ethnicity, higher histological grade, longer tumor length, number of involved lymph nodes, and primary tumor site in the lower esophagus and abdomen were associated with poorer OS (15). Moreover, Tachibana et al. demonstrated an association between differentiation grade and prognosis, with poor cellular differentiation leading to a poor oncologic stage at diagnosis (16). Further research is needed to better understand the impact of these factors on esophageal cancer prognosis.
Key findings
Our study investigated prognostic factors for OS in patients with stage II and III esophageal cancer who underwent neo-CRT followed by surgery. Our findings showed that sex, age, cN category, radiotherapy dose, and hospital volume were independent prognostic factors for OS. Although a trend was observed, we did not find a significant difference in 5-year OS between cT4 (tumor invading adjacent structures) and cT2 (tumor invading muscularis propria; P=0.051) cancer or between poor and moderate cell differentiation (P=0.938). Similarly, we observed a trend but not a significant difference in OS between a BMI of <18.5 kg/m2 and that of 18.5–24 kg/m2 (P=0.052). It should be noted that our study only included patients with stage II or III cancer who underwent neo-CRT and surgery, and did not investigate the impact of BMI, T stage, and cell differentiation on OS in other stages or treatment modalities.
Comparison with similar researches
In comparison with prior research, our study stands out for its larger sample size and use of a national database to compare the survival benefits of different radiotherapy techniques. However, our findings did not indicate a significant difference in survival benefits between rIMRT and IMRT, contrary to the findings of some previous studies.
Two previous studies were reviewed that compared the survival rates of different radiotherapy techniques for patients with esophageal cancer. The first study, conducted by Xu et al., included 195 patients with thoracic esophageal cancer of stage I–II (51%) and stage III–IV (49%) who received concurrent CRT from November 2012 to March 2016. The study compared the OS rates of patients who underwent volumetric-modulated arc therapy (VMAT) and step-and-shoot IMRT (ssIMRT), administered at a dose of 45 to 50.4 Gy. The median follow-up periods were 14.3 months (range, 3.8–34.5 months) for VMAT and 31.8 months (range, 1.8–117.2 months) for ssIMRT. The 2-year OS rates were similar between the two treatment techniques (60.0% for VMAT and 61.4% for ssIMRT; P=0.868). Furthermore, the 2-year recurrence-free survival rates were also similar between the treatment techniques (59.9% for VMAT and 61.8% for ssIMRT; P=0.614) (17). In addition, Yang et al. reported on a study of 78 patients with cervical esophageal cancer of stage I (7%), stage II (32%), stage III (53.8%), and stage IV (6%). The study found no significant differences in survival benefits between radiation techniques. Specifically, the 2-year OS rates associated with three-dimensional conformal radiotherapy (3DCRT), IMRT, and VMAT were 53.6%, 55.6%, and 60.6%, respectively (P=0.965). Moreover, the 2-year failure-free survival rates associated with these techniques were 49.5%, 56.7%, and 60.1% (P=0.998), respectively (18).
Strengths and limitations
Compared to previous studies, our investigation included a larger sample of patients with esophageal cancer who underwent radiotherapy (IMRT, n=1,114; rIMRT, n=487). In addition, our study was the first to use a national database to compare the survival benefits of different radiotherapy techniques. However, our findings did not indicate a significant difference in survival benefits between rIMRT and IMRT, as the median follow-up periods for both techniques were similar (IMRT: 18 months, 95% CI: 9–39; rIMRT: 17 months, 95% CI: 9.5–29.5), and there was no trend for 5-year OS after PSM (P=0.957). Furthermore, there was no significant difference in 5-year OS between the two techniques, regardless of whether IGRT was used (P=0.715). Our study hypothesized that rIMRT could potentially improve prognosis and reduce postoperative mortality, as it can preserve normal tissues and organs. However, our results did not support this hypothesis, as rIMRT did not have an impact on prognosis or reduce postoperative mortality. There are several possible explanations for this finding. Firstly, previous studies have shown that radiotherapy does not increase survival rates in esophageal cancer, even when administered at relatively high doses. Our study found that even the highest dose of rIMRT was higher than that of IMRT (P<0.001; Table S2), and the highest dose of IGRT was also higher than that of non-IGRT (P=0.001; Table S3). However, due to data limitations, we were unable to determine the distribution of side effects. Secondly, we were unable to determine the cause of death, and whether death was caused by radiotherapy-related side effects or the course of the tumor. Previous studies have shown that higher radiation doses (>50.4 Gy) do not increase survival or local/regional control for nonsurgical patients and may even result in higher treatment-related mortality (19). Our data suggest that a neo-CRT dose of >5040 cGy for patients with stage II or III esophageal cancer may result in a significantly lower 5-year OS rate after surgery than a dose of 4,000–5,000 cGy, regardless of the radiotherapy technique used (P=0.001). Studies investigating the impact of hospital volume on esophagectomy outcomes have yielded inconsistent findings (20-23). Some studies have reported a positive association between higher hospital volume and improved OS (20,21), while others have shown that high-volume centers are associated with reduced postoperative length of stay, a cost-related outcome (22). In a study of 11,346 patients who underwent esophageal cancer surgery in 122 hospitals, Kim et al. found that in-hospital mortality rates were significantly higher in low- and medium-volume hospitals than in high-volume hospitals (P<0.001), and patients treated in low- or medium-volume hospitals had lower 5-year OS rates than those treated in high-volume hospitals (P<0.001) (24). Similarly, our study found that patients treated in high-volume hospitals had higher OS rates than those treated in low-volume hospitals (aHR =1.35; 95% CI: 1.05–1.74; P=0.020), but no significant difference in OS rates was observed between medium- and high-volume hospitals (P=0.887).
This study has several limitations that should be considered when interpreting the results. Firstly, the broad range of radiotherapy doses in the database could not be fully explained due to limitations of the data. Secondly, important prognostic factors such as tumor regression grade, ypTN-stage, R0 vs. R1 vs. R2 resection, and pathological complete response data were not available for inclusion in our analysis. Thirdly, we were unable to provide statistical data on posttreatment toxicity due to data limitations.
Explanations of findings
The study’s results suggest that radiotherapy techniques, specifically rIMRT, did not have a significant impact on prognosis or postoperative mortality. Potential explanations for this finding include the fact that radiotherapy, even at relatively high doses, has not been shown to increase survival rates in esophageal cancer. The study also noted that higher doses of radiotherapy did not necessarily translate to improved outcomes. However, data limitations, such as the inability to determine the distribution of side effects and the cause of death, warrant caution in interpreting these findings.
Implications and actions needed
Given the limitations and the reported incidence of complications in patients treated with preoperative CRT followed by surgery for esophageal cancer, future studies should prioritize addressing these gaps and limitations. Previous studies have consistently highlighted the incidence of toxicity and postoperative complications in patients undergoing preoperative CRT followed by surgery for esophageal cancer. Notably, postoperative complications, including pulmonary issues (5.7–48.3%), respiratory failure (5.7–8.3%), pneumonia (20.8%), cardiac issues (5.7–24.1%), and anastomotic leakage (2.9–22%), have been reported in 22.8–49% of cases (5,25-29). Therefore, future studies should address these limitations comprehensively and incorporate relevant prognostic factors and toxicity data. This approach will contribute to a more thorough understanding of the impact of radiotherapy techniques on the outcomes of esophageal cancer treatment.
Conclusions
Our analysis of NHIRD data revealed several independent prognostic factors for OS in stage II and stage III esophageal cancer patients treated with neo-CRT followed by surgery, including sex, age, cN category, radiation dose, and hospital volume. Furthermore, we found no significant difference in OS between patients treated with IMRT and rIMRT. While our study sheds light on the importance of these factors in predicting survival outcomes, future research is needed to further elucidate the key determinants of survival in esophageal cancer patients.
Acknowledgments
We thank all the members of the Department of Radiation Oncology of Chung Shan Medical University Hospital for supporting, including Shao-Ti Lee, Hsi-Chang Chang, Wan-Xun Wang, Xin-Yu Lin, Pei-Fang Cai, Hui-Fang Yang, Ya-Fang Ke, Xiu-Man Zhan, Qi-Wei Chen, Wen-Lan Wang, Yan-Ru Chang, Yan-Mei Huang, Yu-Zhen Xie, Yu-Chan Lu, Shi-Ming Fang, Hsiao-Ju Huang, Ya-Han Chang, Zhe-Ming Chang, Yen-Yu Lo, Qi-Xiang Qu, Jiun-hui Wu, Xuan Chang, Shun-Ri Chang, Li-Chu Lin, Huai-Xuan Chang, Jie Chang.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tro.amegroups.com/article/view/10.21037/tro-23-17/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tro.amegroups.com/article/view/10.21037/tro-23-17/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Human Research Ethics Committee of the Institutional Review Board of Chung Shan Medical University Hospital (CS2-20036). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- National Health Insurance Annual Report 2014-2015. Tapei: National Health Insurance Administration Ministry of Health and Welfare; 2014.
- Chen HS, Hung WH, Ko JL, et al. Impact of Treatment Modalities on Survival of Patients With Locoregional Esophageal Squamous-Cell Carcinoma in Taiwan. Medicine (Baltimore) 2016;95:e3018. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Almhanna K, et al. Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw 2015;13:194-227. [Crossref] [PubMed]
- Reynolds JV, Preston SR, O'Neill B, et al. Neo-AEGIS (Neoadjuvant trial in Adenocarcinoma of the Esophagus and Esophago-Gastric Junction International Study): Preliminary results of phase III RCT of CROSS versus perioperative chemotherapy (Modified MAGIC or FLOT protocol). (NCT01726452). J Clin Oncol 2021;39:4004. [Crossref]
- Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. [Crossref] [PubMed]
- Chen YJ, Liu A, Han C, et al. Helical tomotherapy for radiotherapy in esophageal cancer: a preferred plan with better conformal target coverage and more homogeneous dose distribution. Med Dosim 2007;32:166-71. [Crossref] [PubMed]
- Hsieh CY, Su CC, Shao SC, et al. Taiwan's National Health Insurance Research Database: past and future. Clin Epidemiol 2019;11:349-58. [Crossref] [PubMed]
- Chiang CJ, You SL, Chen CJ, et al. Quality assessment and improvement of nationwide cancer registration system in Taiwan: a review. Jpn J Clin Oncol 2015;45:291-6. [Crossref] [PubMed]
- Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med 2014;33:1242-58. [Crossref] [PubMed]
- Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083-107. [Crossref] [PubMed]
- Andreollo NA, Coelho Neto Jde S, Calomeni GD, et al. Total esophagogastrectomy in the neoplasms of the esophagus and esofagogastric junction: when must be indicated? Rev Col Bras Cir 2015;42:360-5. [Crossref] [PubMed]
- Pereira MR, Lopes LR, Andreollo NA. Quality of life of esophagectomized patients: adenocarcinoma versus squamous cell carcinoma. Rev Col Bras Cir 2013;40:3-9. [Crossref] [PubMed]
- Yuequan J, Shifeng C, Bing Z. Prognostic factors and family history for survival of esophageal squamous cell carcinoma patients after surgery. Ann Thorac Surg 2010;90:908-13. [Crossref] [PubMed]
- Tustumi F, Kimura CM, Takeda FR, et al. Prognostic factors and survival analysis in esophageal carcinoma. Arq Bras Cir Dig 2016;29:138-41. [Crossref] [PubMed]
- Eloubeidi MA, Desmond R, Arguedas MR, et al. Prognostic factors for the survival of patients with esophageal carcinoma in the U.S.: the importance of tumor length and lymph node status. Cancer 2002;95:1434-43. [Crossref] [PubMed]
- Tachibana M, Dhar DK, Kinugasa S, et al. Esophageal cancer patients surviving 6 years after esophagectomy. Langenbecks Arch Surg 2002;387:77-83. [Crossref] [PubMed]
- Xu C, Xi M, Komaki R, et al. Dosimetric and clinical outcomes after volumetric modulated arc therapy for carcinoma of the thoracic esophagus. Adv Radiat Oncol 2017;2:325-32. [Crossref] [PubMed]
- Yang H, Feng C, Cai BN, et al. Comparison of three-dimensional conformal radiation therapy, intensity-modulated radiation therapy, and volumetric-modulated arc therapy in the treatment of cervical esophageal carcinoma. Dis Esophagus 2017;30:1-8. [PubMed]
- Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167-74. [Crossref] [PubMed]
- Fuchs HF, Harnsberger CR, Broderick RC, et al. Mortality after esophagectomy is heavily impacted by center volume: retrospective analysis of the Nationwide Inpatient Sample. Surg Endosc 2017;31:2491-7. [Crossref] [PubMed]
- Metzger R, Bollschweiler E, Vallböhmer D, et al. High volume centers for esophagectomy: what is the number needed to achieve low postoperative mortality? Dis Esophagus 2004;17:310-4. [Crossref] [PubMed]
- Giwa F, Salami A, Abioye AI. Hospital esophagectomy volume and postoperative length of stay: A systematic review and meta-analysis. Am J Surg 2018;215:155-62. [Crossref] [PubMed]
- Kozower BD, Stukenborg GJ. Hospital esophageal cancer resection volume does not predict patient mortality risk. Ann Thorac Surg 2012;93:1690-6; discussion 1696-8. [Crossref] [PubMed]
- Kim BR, Jang EJ, Jo J, et al. The association between hospital case-volume and postoperative outcomes after esophageal cancer surgery: A population-based retrospective cohort study. Thorac Cancer 2021;12:2487-93. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462-7. [Crossref] [PubMed]
- Apinop C, Puttisak P, Preecha N. A prospective study of combined therapy in esophageal cancer. Hepatogastroenterology 1994;41:391-3. [PubMed]
- Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol 2005;6:659-68. [Crossref] [PubMed]
- Wang J, Wei C, Tucker SL, et al. Predictors of postoperative complications after trimodality therapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2013;86:885-91. [Crossref] [PubMed]
Cite this article as: Chang BJ, Lee YC, Chang ST, Huang JY, Chen HL, Tseng HC, Hwang YT, Chou YH. The prognostic value of two different radiotherapy techniques in esophageal cancer patients treated with neoadjuvant chemoradiotherapy. Ther Radiol Oncol 2023;7:19.