
Theranostics potential of somatostatin receptor scintigraphy in both small and non-small cell lung cancers: a preliminary study
Highlight box
Key findings
• In this study, somatostatin receptor (SSTR) expression was observed in all types of lung cancer (LC), including small-cell LC (SCLC) and non-SCLC (NSCLC), using SSTR scintigraphy (SRS) with 99mTc-octreotide. Therefore, SRS can be considered an effective and non-invasive imaging method for accurately staging primary LC, with high accuracy, sensitivity, and specificity. Additionally, peptide receptor radionuclide therapy (PRRT) could be an alternative treatment option for LC patients. However, further studies with a larger sample size are necessary to validate these findings.
What is known and what is new?
• According to previous studies, SSTR expression has been confirmed in SCLC, but there is limited research on NSCLC.
• SSTR expression was observed in both SCLC and NSCLC. Therefore, SRS and PRRT can be considered non-invasive options for managing LC.
What are the implications, and what should change now?
• Due to the high SSTR expression observed with SRS using 99mTc-octreotide, PRRT could be considered as an alternative therapeutic option for LC patients. However, further studies with a larger sample size are needed to confirm these findings.
Introduction
Primary lung cancer (LC), also known as lung carcinoma, is a malignancy of the lung that is classified into two main types: small-cell LC (SCLC) and non-SCLC (NSCLC). SCLC is the most aggressive and accounts for about 10–15% of all cases of LC. NSCLC, which is the most common type of LC, accounts for about 85% of all cases and is further classified into adenocarcinomas, squamous cell carcinomas (SCCs), and large cell carcinomas (LCCs). The most common available treatment procedures for managing LC are surgery, radiotherapy, and chemotherapy (1). The overall 5-year relative survival rate for LC is 15.9%. However, this rate increases to about 50% for patients in the early stages of primary LC without lymph node involvement and metastasis. Therefore, early diagnosis is crucial in the management of patients (2).
Anatomical imaging modalities, such as chest X-ray, magnetic resonance imaging (MRI), and computed tomography (CT), are usually used for the evaluation of LC. While these modalities are considered the first choice in suspected LC, they only provide morphological features of lung lesions and show slight biological features. Therefore, they have a high percentage of equivocal diagnosis. As a result, researchers are focusing on molecular imaging methods, such as radiopharmaceuticals, which can target malignant lung lesions (3,4).
18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) has widely been used for the diagnosis and follow-up of patients with LC. However, due to its high cost and unavailability, inflammatory and infectious pulmonary diseases can show 18F-FDG uptake, leading to false positive results. Another radiopharmaceutical used for the diagnosis of LC is 99mTc-labeled hexakis-2-methoxyisobutylisonitrile (99mTc-MIBI). This radiopharmaceutical has shown a high detection rate (about 90%) and sensitivity (85–90%) as well as a specificity of 75–100% (5,6).
According to the previous studies, expression of somatostatin receptors (SSTRs) has been shown on the surface of some LC cells (3,7-9). Naturally, SSTRs are expressed in neuroendocrine tumors (NETs) (7,10). Due to expression of SSTRs on some LC cells, the use of radiolabeled SSTR analogues as a diagnostic and therapeutic option has been evaluated in the previous studies. Although the use of radiolabeled SSTR analogues, clinically, may have no significant difference in detection of lung malignancy in comparison with other radiopharmaceuticals and even other imaging modalities, they have an advantage, which is their theranostics features i.e., both therapeutic and diagnostic radiotracers can be radiolabeled to SSTR analogues and targeted radionuclide therapy can be performed for the previously imaged disease simultaneously (11). Several radiolabeled SSTR analogues have been assessed including 99mTc-depreotide (12), 111In-pentetreotide (3), 99mTc-octreotide (13), and newly, 68Ga-DOTATATE (14) as diagnostic agents, and 188Re-depreotide, 177Lu-DOTATATE (15) as therapeutic agents.
The aim of this study is to evaluate the effectiveness of SSTR scintigraphy (SRS) with 99mTc-octreotide as a diagnostic SSTR analogue for staging primary LC patients with positive contrast-enhanced CT (CECT) and histologically proven LC. We present this article in accordance with the STARD reporting checklist (available at https://tro.amegroups.com/article/view/10.21037/tro-23-22/rc).
Methods
Between June 2018 and December 2020, 28 primary LC patients who tested positive for CECT and were histologically diagnosed with LC underwent SRS. The patients were classified into SCLC and NSCLC based on histopathologic results. NSCLC patients were further classified into SCC, adenocarcinoma, and LCC. All patients were in the staging step and did not undergo any conventional therapy. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and approved by the Ethics Committee of Bushehr University of Medical Sciences (IR.BPUMS.REC.1398.083). Informed consent was obtained from patients.
Chest CT
All patients underwent CECT (Lightspeed 16, GE Medical Systems, GE Healthcare, Anaheim, CA, USA) with the usual protocol [collimation 16 mm × 1.5 mm, reconstruction interval (RI) =1 mm, reconstruction width (RW) =2 mm, 120 kV, 150 mAs, rotation time 0.75 seconds, pitch 1.5] within 2 weeks before 99mTc-octreotide scintigraphy. Contrast agent was injected at 2 mL/kg body weight at a rate of 4 mL/s. The chest scan was started 35 seconds after the contrast injection. The images were reviewed by two expert radiologists.
99mTc-octreotide scintigraphy
For SRS, 99mTc-octreotide (Pars Isotope Co., Tehran, Iran) was prepared according to the manufacturer’s instructions. Scintigraphy was acquired within 2 to 4 hours after intravenous injection of 740 MBq (20 mCi) 99mTc-octreotide with a dual-head gamma camera [Philips (ADAC) Vertex Plus, Milpitas, CA, USA] equipped with a low-energy high-resolution collimator, and the energy window was set at 140 keV ±20%. All patients underwent examination with a whole-body scan (WBS) and chest SPECT. WBS was acquired in anterior and posterior views with a matrix size of 256×1,024 and a speed of 15 cm/min. The single-photon emission CT (SPECT) was acquired with a matrix size of 128×128 in 360-degree rotation and 64 projections (20 seconds/projection). Image reconstruction was performed using an iterative algorithm without attenuation correction.
All images were reviewed by two nuclear medicine specialists. Any non-physiological and focal radiotracer accumulation higher than the background was considered a suspicious LC lesion. For semi-quantitative analysis of SRS images, the Krenning score (KS) was used, which is ranged from 0–4 (0, none; 1, much lower than the liver; 2, slightly less than or equal to the liver; 3, greater than the liver; 4, greater than the spleen) according to the radiotracer uptake in the lesion.
Image interpretation
To compare CECT and SRS images, the lung was divided into five portions, including the right upper lobe (RUL), right middle lobe (RML), right lower lobe (RLL), left upper lobe (LUL), and left lower lobe (LLL). Each lung portion was considered positive if a non-physiological mass in CT or radiotracer uptake in scintigraphy was observed.
Statistical analysis
All data were presented as median with a range. The chi-square test was used to evaluate categorical variables, and a P value <0.05 was considered statistically significant. The Wilcoxon test was used to assess statistical significance between groups. The values of accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) in different lobes of the lung were calculated for 99mTC-octreotide scintigraphy (the result of CECT was considered as the gold standard). Data analysis was performed using SPSS (Windows software version 20, SPSS, IBM Corp., Armonk, NY, USA).
Results
In this study, 28 histopathologically proven primary LC patients with positive CECT and a median age of 74.5 (range, 48–90) years, including 17 males (60.7%) and 11 females (39.3%), underwent SRS with 99mTc-octreotide. According to the histopathology results, out of 28 patients, 3 cases were SCLC and 25 cases were NSCLC, of which 19 cases were SCC, and 6 cases were adenocarcinomas. According to CT results, the median diameter of tumors was 5 cm with a range of 2–9 cm. Out of 28 cases, 26 patients (93%) showed positive SRS. One case with adenocarcinoma and one with SCC showed normal SRS. The median KS was 1 with a range of 1–2. Baseline characteristics of the patients are presented in Table 1.
Table 1
| Characteristics | Value (n=28) |
|---|---|
| Sex, n (%) | |
| Male | 17 (60.7) |
| Female | 11 (39.3) |
| Age (years) | |
| Median | 74.5 |
| Range | 48–90 |
| Type of cancer, n (%) | |
| SCLC | 3 (10.7) |
| NSCLC | 25 (89.3) |
| SCC | 19 (67.9) |
| Adenocarcinoma | 6 (21.4) |
| KS | |
| Median | 1 |
| Range | 1–2 |
| Tumor size (cm) | |
| Median | 5 |
| Range | 2–9 |
SCLC, small-cell lung cancer; NSCLC, non-small-cell lung cancer; SCC, squamous cell carcinoma; KS, Krenning score.
Table 2 shows the detailed comparison of CECT and SRS in different lobes of the lung, which showed no significant difference (P>0.05). In RUL, out of 28 cases, 27 cases showed the same results in both modalities, but one case showed positive CECT and negative SRS. In LUL, both modalities showed the same results in all cases. In RML, three cases showed negative CECT and positive scintigraphy, and one case showed positive CT and negative SRS. In RLL, one case showed negative CECT and positive scintigraphy, and one case showed positive CECT and negative scintigraphy. Finally, in LLL, one case showed negative CECT and positive scintigraphy. Additionally, Table 3 showed the values of accuracy, sensitivity, specificity, PPV, and NPV in different lobes of the lung for 99mTC-octreotide scintigraphy.
Table 2
| Scintigraphy | CECT | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RUL | RML | RLL | LUL | LLL | ||||||||||
| Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | |||||
| Positive | 6 | 0 | 3 | 3 | 7 | 1 | 9 | 0 | 2 | 1 | ||||
| Negative | 1 | 21 | 1 | 21 | 1 | 19 | 0 | 19 | 0 | 25 | ||||
CECT, contrast-enhanced computed tomography; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe.
Table 3
| Value | RUL | LUL | RML | RLL | LLL |
|---|---|---|---|---|---|
| Accuracy (%) | 96 | 100 | 88 | 93 | 96 |
| Sensitivity (%) | 88 | 100 | 80 | 89 | 100 |
| Specificity (%) | 100 | 100 | 89 | 95 | 96 |
| PPV (%) | 100 | 100 | 57 | 88 | 66 |
| NPV (%) | 95 | 100 | 96 | 95 | 100 |
PPV, positive predictive value; NPV, negative predictive value; RUL, right upper lobe; LUL, left upper lobe; RML, right middle lobe; RLL, right lower lobe; LLL, left lower lobe.
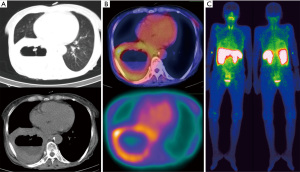
Furthermore, no significant difference was obtained in evaluating the effect of several factors, including age, sex, tumor size, and type of tumor on scintigraphy results and KS (P>0.05). Figure 1 presents the images of a patient with SCC.
Discussion
In recent years, the development and use of theranostic agents in oncology has increased for the management of several cancer types. Numerous theranostic agents have been studied in the last few decades. Radiolabeled SSTR analogues, such as 68Ga/177Lu-DOTATATE, are one of the most common theranostic agents for the management of NETs. This approach has become a promising method with high detection rates and therapy efficacy in recent years (16-19). In addition to NETs, various studies have been performed on the use of SSTR analogues in the management of non-NET tumors such as medullary thyroid cancer, neuroblastoma, and glioblastoma, with low toxicity (10,20-27).
Various studies have suggested that LC cell might generate and secrete somatostatin (3,12,28-30), therefore, we performed a study on the expression of SSTR for staging various types of LC, including SCLC and NSCLC with positive CECT and proven histopathology. For the evaluation of SSTR expression, all patients underwent 99mTc-octreotide scintigraphy. The lung was classified into five main lobes: RUL, RML, RLL, LUL, and LLL. Then, the results of the scintigraphy were compared with CECT for each lobe. In our experience, all 99mTc-octreotide scans were positive irrespective of the histological type of the tumor and whether or not they were NET. The ranges of accuracy, sensitivity, specificity, PPV, and NPV of scintigraphy in each lobe were obtained and were 88–100%, 80–100%, 89–100%, 57–100%, and 95–100%, respectively. According to the previous studies, the accuracy and sensitivity of SRS compared to CT were about 65% (3) and 90–100% (4,31) for primary lung tumors, respectively. Wang et al. evaluated the clinical efficacy of a combination of CT and SRS with 99mTc-octreotide in differentiating cancer from benign pulmonary nodules (9). They showed that 99mTc-octreotide uptake was significantly higher in LC compared to benign lesions. The specificity of SRS was 72.7% compared to 63.6% for CT for the diagnosis of pulmonary malignant tumors. When both modalities were used in combination, the specificity was 81.8% (9). Axelsson et al. evaluated the diagnostic value of SRS with 99mTc-depreotide in suspected LC patients (28). Of the 99 evaluated patients, SRS was positive in 62 out of 66 malignancies but was false positive in 16 out of 33 patients with benign lesions. The sensitivity and specificity of 99mTc-depreotide were 98% and 52%, respectively, for LC (28).
Nowadays, 18F-FDG PET is widely utilized for the diagnosis of LC. In a study, the clinical efficacy of SRS with 99mTc-octreotide was compared to 18F-FDG PET in the detection of patients with suspected LC. It was indicated that the sensitivity of both 99mTc-octreotide and 18F-FDG was 100%. However, the specificity, PPV, and NPV of 99mTc-octreotide were 75.7%, 90.1%, and 100%, respectively, while those of 18F-FDG were 46.1%, 83.8%, and 100%, respectively (13). The uptake of 18F-FDG in inflammatory and infectious lung diseases in an important disadvantage. According to the mentioned study, SRS showed higher uptake in malignancy compared to benign lesions (13), which could be the advantage of SRS over 18F-FDG for diagnosis of LC.
There are several studies in use of various theranostic agents including chemokine receptor CXCR4 (32), fibroblast activation protein inhibitor (FAPI) (33,34) and SSTR analogues (14,35,36) for management of patients with LC. In a study, the feasibility of CXCR4 in SCLC patients was evaluated by using 68Ga-pentaxifor PET/CT in comparison with 18F-FDG PET/CT and 68Ga-DOTATATE PET/CT. 68Ga-pentaxifor was positive in 80% of patients and showed more lesions with significantly higher tumor-to-background ratios in comparison with 68Ga-DOTATATE PET/CT. Of six patients had both 68Ga-pentaxifor PET/CT and 18F-FDG PET/CT, two patients who were positive on 18F-FDG PET were missed by 68Ga-pentaxifor PET/CT and in the remainder 68Ga-pentixafor detected an equal (n=2) or higher (n=2) number of lesions (32).
In the view of theranostics with SSTR analogues, there are several studies on the use of these as theranostics agents for diagnosis and therapy of several non-gastroenteropancreatic NETs (non-GEP-NETs) with high SSTR expression. We previously reported acceptable efficacy of 68Ga/177Lu-DOTATATE for management of patients with Non-GEP-NETs including neuroblastoma (22), prostate cancer (10), high-grade glioma (25,26), meningioma (21), and pituitary adenoma (20) with low toxicity (23,24). Therefore, one of most important advantages of SSTR analogues compared to other diagnostic modalities is their theranostics feature. According to the results of our study indicating uptake of 99mTc-octreotide and expression of SSTR in all types of primary LCs, therapeutic SSTR analogues such as 177Lu-DOTATATE can be considered as an alternative option in these patients specially in the cases who are refractory to conventional treatments. In a study, Lapa et al. evaluated SSTR expression in SCLC with 68Ga-DOTATATE PET/CT revealed that PET/CT was positive in 47% of patients (36). In addition, peptide receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE was performed for four patients with intensive SSTR expression resulted in one partial response and one stable disease (36). Mirvis et al. evaluated efficacy of PRRT with 90Y-DOTATATE and/or 177Lu-DOTATATE in advanced metastatic bronchial NETs demonstrating partial response in 10 patients (40%), stable disease in 12 patients (48%) and progressive disease in 2 patients (8%) (37). A further patient (5%) died after two cycles without any follow-up scan and is also classified as having progressed (37).
Our study has several limitations. First, the patients should be classified into different groups according to their cancer types for evaluation of SSTR expression but it was impossible due to low sample size. Second, lack of SPECT/CT for better anatomical assessment. Third, unavailability of PET is another limitation of our study because in the view of theranostics, for more accurate evaluation of SSTR expression in primary LC, it is better to use 68Ga-DOTATATE instead of SRS since the low spatial resolution of gamma camera may cause lesions less than 2 cm in diameter not detected in planar imaging, specially.
Conclusions
As radiotracer uptake was observed in all types of primary LC, it can be concluded that SRS with 99mTc-octreotide is an effective non-invasive imaging modality for diagnosing primary LC with high accuracy, sensitivity, and specificity. Due to the high SSTR expression in LC according to SRS with 99mTc-octreotide, PRRT could be an alternative therapeutic option for LC patients. However, further studies with a larger sample size are needed.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tro.amegroups.com/article/view/10.21037/tro-23-22/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tro.amegroups.com/article/view/10.21037/tro-23-22/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and approved by the Ethics Committee of Bushehr University of Medical Sciences (IR.BPUMS.REC.1398.083). Informed consent was obtained from patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018;553:446-54. [Crossref] [PubMed]
- van Rens MT, de la Rivière AB, Elbers HR, et al. Prognostic assessment of 2,361 patients who underwent pulmonary resection for non-small cell lung cancer, stage I, II, and IIIA. Chest 2000;117:374-9. [Crossref] [PubMed]
- Genestreti G, Bongiovanni A, Burgio MA, et al. 111In-Pentetreotide (OctreoScan) scintigraphy in the staging of small-cell lung cancer: its accuracy and prognostic significance. Nucl Med Commun 2015;36:135-42. [Crossref] [PubMed]
- Semprebene A, Ferraironi A, Franciotti G, et al. 111In-octreotide scintigraphy in small cell lung cancer. Q J Nucl Med 1995;39:108-10. [PubMed]
- Minai OA, Raja S, Mehta AC, et al. Role of Tc-99m MIBI in the evaluation of single pulmonary nodules: a preliminary report. Thorax 2000;55:60-2. [Crossref] [PubMed]
- Nikoletic K, Lucic S, Peter A, et al. Lung 99mTc-MIBI scintigraphy: impact on diagnosis of solitary pulmonary nodule. Bosn J Basic Med Sci 2011;11:174-9. [Crossref] [PubMed]
- Kuyumcu S, Adalet I, Sanli Y, et al. Somatostatin receptor scintigraphy with 111In-octreotide in pulmonary carcinoid tumours correlated with pathological and 18FDG PET/CT findings. Ann Nucl Med 2012;26:689-97. [Crossref] [PubMed]
- Tzannou IA, Karapanagiotou EM, Charpidou A, et al. The use of radiolabeled somatostatin analog scintigraphy in the staging of small cell lung cancer patients. Am J Clin Oncol 2007;30:503-6. [Crossref] [PubMed]
- Wang L, Yin X, Wang F, et al. The usefulness of combined diagnostic CT and (99m)Tc-octreotide somatostatin receptor SPECT/CT imaging on pulmonary nodule characterization in patients. Cancer Biother Radiopharm 2013;28:731-6. [Crossref] [PubMed]
- Assadi M, Pirayesh E, Rekabpour SJ, et al. 177Lu-PSMA and 177Lu-DOTATATE Therapy in a Patient With Metastatic Castration-Resistant Prostate Cancer and Neuroendocrine Differentiation. Clin Nucl Med 2019;44:978-80. [Crossref] [PubMed]
- Pencharz D, Gnanasegaran G, Navalkissoor S. Theranostics in neuroendocrine tumours: somatostatin receptor imaging and therapy. Br J Radiol 2018;91:20180108. [Crossref] [PubMed]
- Apostolopoulos DJ, Koletsis EN, Spyridonidis T, et al. Tc-99m depreotide SPECT/CT for lymph node staging of non-small-cell lung cancer. Ann Nucl Med 2014;28:463-71. [Crossref] [PubMed]
- Wang F, Wang Z, Yao W, et al. Role of 99mTc-octreotide acetate scintigraphy in suspected lung cancer compared with 18F-FDG dual-head coincidence imaging. J Nucl Med 2007;48:1442-8. [Crossref] [PubMed]
- Walker R, Deppen S, Smith G, et al. 68Ga-DOTATATE PET/CT imaging of indeterminate pulmonary nodules and lung cancer. PLoS One 2017;12:e0171301. [Crossref] [PubMed]
- Kim C, Liu SV, Subramaniam DS, et al. Phase I study of the (177)Lu-DOTA(0)-Tyr(3)-Octreotate (lutathera) in combination with nivolumab in patients with neuroendocrine tumors of the lung. J Immunother Cancer 2020;8:e000980. [Crossref] [PubMed]
- Kalantarhormozi M, Hassanzadeh S, Rekabpour SJ, et al. Peptide Receptor Radionuclide Therapy Using (177) Lu-DOTATATE in Advanced Neuroendocrine Tumors (NETs) in a Limited-Resource Environment. World J Nucl Med 2022;21:215-21. [Crossref] [PubMed]
- Basu S, Fargose P. 177Lu-DOTATATE PRRT in Recurrent Skull-Base Phosphaturic Mesenchymal Tumor Causing Osteomalacia: A Potential Application of PRRT Beyond Neuroendocrine Tumors. J Nucl Med Technol 2016;44:248-50. [Crossref] [PubMed]
- Mirzaei S, Bastati B, Lipp RW, et al. Additional lesions detected in therapeutic scans with 177Lu-DOTATATE reflect higher affinity of 177Lu-DOTATATE for somatostatin receptors. Oncology 2011;80:326-9. [Crossref] [PubMed]
- Thapa P, Ranade R, Ostwal V, et al. Performance of 177Lu-DOTATATE-based peptide receptor radionuclide therapy in metastatic gastroenteropancreatic neuroendocrine tumor: a multiparametric response evaluation correlating with primary tumor site, tumor proliferation index, and dual tracer imaging characteristics. Nucl Med Commun 2016;37:1030-7. [Crossref] [PubMed]
- Assadi M, Nemati R, Shooli H, et al. An aggressive functioning pituitary adenoma treated with peptide receptor radionuclide therapy. Eur J Nucl Med Mol Imaging 2020;47:1015-6. [Crossref] [PubMed]
- Assadi M, Rekabpour SJ, Amini A, et al. Peptide Receptor Radionuclide Therapy with (177)Lu-DOTATATE in a Case of Concurrent Neuroendocrine Tumors and Meningioma: Achieving Two Things in a Single Action. Mol Imaging Radionucl Ther 2021;30:107-9. [Crossref] [PubMed]
- Fathpour G, Jafari E, Hashemi A, et al. Feasibility and Therapeutic Potential of Combined Peptide Receptor Radionuclide Therapy With Intensive Chemotherapy for Pediatric Patients With Relapsed or Refractory Metastatic Neuroblastoma. Clin Nucl Med 2021;46:540-8. [Crossref] [PubMed]
- Jafari E, Ahmadzadehfar H, Bagheri D, et al. Assessment of early oxidative stress following the use of radiotheranostics agents 177Lu-PSMA for prostate cancer and 177Lu-DOTATATE for neuroendocrine tumors; radioprotective effect of vitamin C. Nucl Med Commun 2021;42:325-31. [Crossref] [PubMed]
- Jafari E, Amini AL, Ahmadzadehfar H, et al. Cardiotoxicity and cardiac monitoring following the use of radiotheranostics agents including 177Lu-PSMA for prostate cancer and 177Lu-DOTATATE for neuroendocrine tumors. Nuklearmedizin 2021;60:99-105. [Crossref] [PubMed]
- Nemati R, Shooli H, Rekabpour SJ, et al. Feasibility and Therapeutic Potential of Peptide Receptor Radionuclide Therapy for High-Grade Gliomas. Clin Nucl Med 2021;46:389-95. [Crossref] [PubMed]
- Shooli H, Nemati R, Ahmadzadehfar H, et al. Theranostics in Brain Tumors. PET Clin 2021;16:397-418. [Crossref] [PubMed]
- Dadgar H, Jafari E, Ahmadzadehfar H, et al. Feasibility and therapeutic potential of the 68Ga/177Lu-DOTATATE theranostic pair in patients with metastatic medullary thyroid carcinoma. Ann Endocrinol (Paris) 2023;84:45-51. [Crossref] [PubMed]
- Axelsson R, Herlin G, Bååth M, et al. Role of scintigraphy with technetium-99m depreotide in the diagnosis and management of patients with suspected lung cancer. Acta Radiol 2008;49:295-302. [Crossref] [PubMed]
- Harders SW, Madsen HH, Hjorthaug K, et al. Limited value of 99mTc depreotide single photon emission CT compared with CT for the evaluation of pulmonary lesions. Br J Radiol 2012;85:e307-13. [Crossref] [PubMed]
- Herlin G, Kölbeck KG, Menzel PL, et al. Quantitative assessment of 99mTc-depreotide uptake in patients with non-small-cell lung cancer: immunohistochemical correlations. Acta Radiol 2009;50:902-8. [Crossref] [PubMed]
- Bohuslavizki KH, Brenner W, Günther M, et al. Somatostatin receptor scintigraphy in the staging of small cell lung cancer. Nucl Med Commun 1996;17:191-6. [Crossref] [PubMed]
- Lapa C, Lückerath K, Rudelius M, et al. [68Ga]Pentixafor-PET/CT for imaging of chemokine receptor 4 expression in small cell lung cancer--initial experience. Oncotarget 2016;7:9288-95. [Crossref] [PubMed]
- Wang L, Tang G, Hu K, et al. Comparison of (68)Ga-FAPI and (18)F-FDG PET/CT in the Evaluation of Advanced Lung Cancer. Radiology 2022;303:191-9. [Crossref] [PubMed]
- Assadi M, Rekabpour SJ, Jafari E, et al. Feasibility and Therapeutic Potential of 177Lu-Fibroblast Activation Protein Inhibitor-46 for Patients With Relapsed or Refractory Cancers: A Preliminary Study. Clin Nucl Med 2021;46:e523-30. [Crossref] [PubMed]
- Deppen SA, Blume J, Bobbey AJ, et al. 68Ga-DOTATATE Compared with 111In-DTPA-Octreotide and Conventional Imaging for Pulmonary and Gastroenteropancreatic Neuroendocrine Tumors: A Systematic Review and Meta-Analysis. J Nucl Med 2016;57:872-8. [Crossref] [PubMed]
- Lapa C, Hänscheid H, Wild V, et al. Somatostatin receptor expression in small cell lung cancer as a prognostic marker and a target for peptide receptor radionuclide therapy. Oncotarget 2016;7:20033-40. [Crossref] [PubMed]
- Mirvis E, Toumpanakis C, Mandair D, et al. Efficacy and tolerability of peptide receptor radionuclide therapy (PRRT) in advanced metastatic bronchial neuroendocrine tumours (NETs). Lung Cancer 2020;150:70-5. [Crossref] [PubMed]
Cite this article as: Bahtouee M, Jafari E, Salehahmadi F, Khazaei M, Assadi M. Theranostics potential of somatostatin receptor scintigraphy in both small and non-small cell lung cancers: a preliminary study. Ther Radiol Oncol 2023;7:18.


