
Decline of circulating monocyte and neutrophil counts in COVID-19-infected patients with cancer receiving radiotherapy
Highlight box
Key findings
• The decline in circulation monocytes and neutrophils was more vigorous in cancer patients receiving radiotherapy (RT) with synchronous coronavirus 2/coronavirus disease 2019 (COVID-19) infection compared with that in COVID-naive patients.
What is known and what is new?
• Many studies have shown that monocytes play an important role in causing inflammation in COVID-infected patients. However, due to the radiosensitivity of hematopoietic stem cells, the absolute monocyte count decreases during RT or concurrent chemoRT course. In our manuscript, the absolute monocytes and neutrophils counts were significantly decreased during RT compared with the data collected before RT in COVID-infected patients.
What is the implication, and what should change now?
• The results may provide an important reference for evaluating impact of COVID-19 infection on complete blood count with differential count in cancer patients receiving RT.
Introduction
Circulating blood monocytes are produced by hematopoiesis in the bone marrow and recruited to tissues to transform into resident macrophages. Tissue recruitment of circulating monocytes occurs in response to the increased demand following tissue inflammation, infection, and malignancy. Classically, monocytes are phagocytic innate immune cells that are divided into three subsets according to the expression of the surface antigens, CD14 and CD16 [classical (CD14+CD16−), non-classical (CD14dimCD16+), and intermediate (CD14+CD16+)] (1). Under pathological conditions, such as viral infection, monocytes are recruited by inflammatory mediators to infiltrate the affected tissues. Inflammatory macrophages and cell with dendritic cell-like phenotypes fulfill the effector functions, which include pro- and anti-inflammatory activities, antigen presentation, and tissue remodeling (2).
Blood monocytes provide a narrow window for the systemic immune response, from production to tissue recruitment, reflecting the impact of the infection on the host. Monocyte-derived macrophages are the main generators of inflammation in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)/coronavirus disease 2019 (COVID-19) (3). Clinical investigations have demonstrated that patients with COVID-19 have inconsistent monocyte counts (3). In a more specific monocyte subset analysis, monocytes in the blood of patients with moderate COVID-19 present an inflammatory, interferon-stimulated gene-driven phenotype. Cellular dysfunction, the dominant feature of disease severity is epitomized by the loss of HLA-DR expression and induction of S100 alarmin expression (4). Flow cytometric analyses of peripheral blood have shown a decline in the percentage of total monocytes in the blood of patients with severe COVID-19 (5,6). Notably, this reduction was observed only transiently in a longitudinal study of immune cells in severe cases, indicating a highly time-sensitive immune response (7). Another study demonstrated that the white blood cell (WBC) count and absolute neutrophil count (ANC), but not lymphocyte and monocyte counts were greater in COVID-19-infected patients in the intensive care unit (ICU) group than those in the non-ICU group (8).
Radiosensitivity of hematopoietic stem cells causes decrease in absolute monocyte count (AMC) during radiotherapy (RT) or concurrent chemoRT (9,10). However, no study has investigated the complete blood count (CBC) of COVID-infected patients during RT, especially the AMC, and as stated above, monocytes play an important role in inflammation in COVID-infected patients.
Therefore, the aim of our study was to explore the differences in AMCs and other cell lineages during RT between COVID-infected and COVID-naive patients. These results may provide an important reference for evaluating the impact of COVID-19 on CBC with differential counts in patients with cancer receiving RT. We present this article in accordance with the STROBE reporting checklist (available at https://tro.amegroups.com/article/view/10.21037/tro-23-14/rc).
Methods
Patient enrollment
This retrospective study enrolled both COVID-infected patients (n=22) and COVID-naive patients (n=22). Both groups received RT from April 2022 to August 2022. The CBC data before and during RT were available for all enrolled patients. Patients without CBC data abovementioned were excluded. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of MacKay Memorial Hospital (No. 23MMHIS154e) and individual consent for this retrospective analysis was waived.
RT technique
All patients were immobilized in the supine position using thermoplastic facial masks or alpha-cradle molds, depending on the site of treatment. Isocenters were marked on the mask or patients’ bodies using laser markers to ensure the reproducibility of daily treatment. Subsequently, the patients underwent simulated computed tomography with a thickness of 3 mm per slice. The dose and fraction of RT were planned according to cancer type, stage, and treatment intention. The patients received RT for 5 consecutive days per week. The RT was designed using an RT planning system (Eclipse version 13.0, Varian, CA, USA; Pinnacle 3 9.10, Philips, WI, USA). All RT treatments were delivered via three-dimensional RT, intensity-modulated RT, or dynamic arc using linear accelerators (Clinac iX, Varian, CA, USA; Synergy, Elekta, Stockholm, Sweden; Versa HD, Stockholm, Sweden).
Data collection and calculation
The CBC data were examined within 1 month before both COVID infection and RT treatment were selected. In contrast, blood tests for the lowest monocyte count during RT were performed. Using the above data, the ratios of neutrophils to lymphocytes (NLR), platelets to lymphocytes (PLR), and monocytes to lymphocytes (MLR) were calculated, respectively, which are biomarkers of inflammation and are considered as predictors of the prognosis of systemic inflammatory diseases, which can even affect the prognosis in patients with cancer, as evidenced in this study (11). In addition, the delta values were calculated as the blood count during RT minus the count before RT.
Polymerase chain reaction (PCR)
All COVID-infected patients in this study were diagnosed by PCR testing, which amplified and detected SARS-CoV-2 RNA in the upper respiratory tract. The PCR sequences detected at that time followed the standards provided by the National Laboratory of Taiwan Centers for Disease Control, Kwen-Yang Laboratory.
Statistics analysis
Descriptive statistical analyses were performed using SPSS software (IBM Corp., Released 2019, Version 24.0, Armonk, NY, USA). To analyze the differences in the blood cell counts of the same patients before and during RT, the paired t-test was used, whereas the unpaired t-test was performed to calculate the variation in blood cell counts between COVID-infected and COVID-naive patients.
Results
This retrospective study enrolled 44 patients between April 2022 and August 2022. The groups were well balanced, with 22 patients in each group. Detailed patient information is provided in the supplementary Information (Tables S1,S2). The median age was 60.5 years. No severe infections were observed in the COVID-infected group. Approximately 43% of the patients received chemotherapy during RT, with no significant distributional deviation in either group (P=0.761). The percentages of patients with and without COVID infection receiving RT doses ≥50 Gy were 68% and 77%, respectively (P=0.498). Detailed patient characteristics are summarized in Table 1.
Table 1
| Characteristics | COVID-infected | COVID-naive | P |
|---|---|---|---|
| Gender, n | 0.757 | ||
| Male | 9 | 8 | |
| Female | 13 | 14 | |
| Age (years), median | 61 | 58 | 0.553 |
| Stage, n | 0.278 | ||
| In situ, I, II | 9 | 13 | |
| III, IV | 11 | 8 | |
| Not applicable | 2 | 1 | |
| Dose (Gy), n | 0.498 | ||
| <50 | 7 | 5 | |
| ≥50 | 15 | 17 | |
| Systemic therapy | 0.761 | ||
| Yes | 10 | 9 | |
| No | 12 | 13 | |
| Antivirus therapy | 0.002 | ||
| Yes | 8 | 0 | |
| No | 14 | 22 |
COVID, coronavirus disease 2019.
Complete blood count profiles in COVID-infected and COVID-naive patients
In COVID-infected patients, the AMC (P<0.001), absolute lymphocyte count (ALC) (P<0.001), ANC (P=0.005), and platelet counts (P=0.015) were significantly decreased, whereas the NLR and PLR increased during RT in comparison with the data before RT, as shown in Figures 1-4. Detailed data of COVID-infected patients are summarized in Table 2. Data from COVID-naive patients revealed a significant decline in AMC, ALC, and platelet count, with a significant increase in NLR, PLR, and MLR (Table 2).


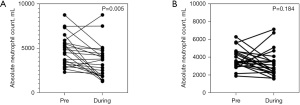

Table 2
| Complete blood count | COVID-infected | COVID-naive | |||
|---|---|---|---|---|---|
| Pre-radiotherapy | During radiotherapy | Pre-radiotherapy | During radiotherapy | ||
| AMC (/μL) | 610.7±237.6 | 318.7±170 (−48%)* | 544.0±154.9 | 374.6±182.8 (−31%)* | |
| ALC (/μL) | 1,468.8±608.6 | 740.2±546.3 (−50%)* | 1,626.3±529.8 | 793.0±493.0 (−51%)* | |
| ANC (/μL) | 4,755.4±1,732.0 | 3,510.1±1,954.9 (−26%)* | 3,812.2±1,130.7 | 3,301.9±1,509.7 (−13%) | |
| PLT (1,000/μL) | 273.9±82.7 | 228.5±72.9 (−17%)* | 244.1±95.9 | 187.8±82.9 (−23%)* | |
| NLR | 4.0±2.5 | 9.8±11.6 (145%)* | 2.7±1.5 | 6.3±5.4 (133%)* | |
| PLR | 248.6±169.3 | 476.0±380.9 (91%)* | 187.9±122.6 | 343.4±229.1 (83%)* | |
| MLR | 0.5±0.4 | 0.7±0.6 (40%) | 0.4±0.3 | 0.6±0.4 (50%)* | |
Data were presented as mean ± standard deviation. The numbers in parentheses indicate the percentage changes before and during RT in the two groups. *, differences in significance (P<0.05). COVID, coronavirus disease; AMC, absolute monocyte count; ALC, absolute lymphocyte count; ANC, absolute neutrophil count; PLT, platelet; NLR, ratio of neutrophils to lymphocytes; PLR, ratio of platelets to lymphocytes; MLR, ratio of monocytes to lymphocytes.
Degree of decline of complete blood count with differential count in COVID-infected and COVID-naive patients
The percentages of change before and during RT in the two groups are shown in Table 2. The degree of decline in differential counts of leukocytes in COVID-infected group was more vigorous than those in COVID-naive group in terms of AMC (−48% versus −31%) and ANC (−26% versus −13%).
Discussion
The issue of COVID-19-infected patients with cancer receiving RT is an emerging concern that lacks sufficient clinical information. In the present study, a more vigorous decline in AMC and ANC was observed, which could provide an immunological reference for managing patients with cancer receiving RT in clinical practice.
The roles of monocytes and monocyte-derived macrophages in COVID-19 are important from an immunological perspective. The hyper-inflammatory response induced by COVID-19 is a major cause of severe disease and mortality. Monocyte-derived and -recruited macrophages are populations of innate immune cells that sense and respond to microbial invasion by producing inflammatory mediators that eliminate microbes and enhance tissue repair. However, a dysregulated macrophage response may damage host tissues under conditions, such as macrophage activation syndrome (12,13). The more vigorous decline in AMC in patients with cancer and synchronous COVID-19 receiving RT indicated the potential role of RT in the management of COVID-19-induced hyper-inflammation. This association requires further investigation, involving the analysis of monocyte subsets in clinical studies and examination of causal relationships in animal experiments.
As for neutrophils, the increase in neutrophils may be related to unfavorable outcome in COVID-19-infected patients as well as patients with cancer and without COVID-19 infection. In adult COVID-19-infected patients with cancer, an elevated NLR is significantly associated with poor survival (14). However, the role of neutrophil count in the prognosis of patients with cancer and synchronous COVID-19 receiving RT remains uncertain. Our results demonstrated a more vigorous decline in ANC in COVID-19-infected patients receiving RT. These data may provide an alert in clinical practice, suggesting the consideration of risks of other infections, such as bacterial infections.
This study had several limitations, including a relatively small sample size and lack of subset analysis for AMC and ANC. Although this study provided clinical information regarding COVID-19-infected patients with cancer receiving RT, further investigations are warranted to validate and confirm these findings.
Conclusions
In conclusion, a more vigorous decline in circulating monocytes and neutrophils was noted in patients with cancer receiving RT for synchronous COVID-19 infection.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tro.amegroups.com/article/view/10.21037/tro-23-14/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tro.amegroups.com/article/view/10.21037/tro-23-14/coif). Y.J.C. serves as the Editor-in-Chief of Therapeutic Radiology and Oncology. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of MacKay Memorial Hospital (No. 23MMHIS154e) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kapellos TS, Bonaguro L, Gemünd I, et al. Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front Immunol 2019;10:2035. [Crossref] [PubMed]
- Guilliams M, Mildner A, Yona S. Developmental and Functional Heterogeneity of Monocytes. Immunity 2018;49:595-613. [Crossref] [PubMed]
- Wang J, Jiang M, Chen X, et al. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol 2020;108:17-41. [Crossref] [PubMed]
- Spinetti T, Hirzel C, Fux M, et al. Reduced Monocytic Human Leukocyte Antigen-DR Expression Indicates Immunosuppression in Critically Ill COVID-19 Patients. Anesth Analg 2020;131:993-9. [Crossref] [PubMed]
- Qin C, Zhou L, Hu Z, et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis 2020;71:762-8. [Crossref] [PubMed]
- Laing AG, Lorenc A, Del Molino Del Barrio I, et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med 2020;26:1623-35. [Crossref] [PubMed]
- Payen D, Cravat M, Maadadi H, et al. A Longitudinal Study of Immune Cells in Severe COVID-19 Patients. Front Immunol 2020;11:580250. [Crossref] [PubMed]
- Pozdnyakova O, Connell NT, Battinelli EM, et al. Clinical Significance of CBC and WBC Morphology in the Diagnosis and Clinical Course of COVID-19 Infection. Am J Clin Pathol 2021;155:364-75. [Crossref] [PubMed]
- Xiang X, Li N, Ding Z, et al. Peripheral Lymphocyte Counts and Lymphocyte-Related Inflammation Indicators During Radiotherapy for Pelvic Malignancies: Temporal Characterization and Dosimetric Predictors. Technol Cancer Res Treat 2022;21:15330338221116494. [Crossref] [PubMed]
- Lin CH, Chou WC, Wu YY, et al. Prognostic significance of dynamic changes in lymphocyte-to-monocyte ratio in patients with head and neck cancer treated with radiotherapy: results from a large cohort study. Radiother Oncol 2021;154:76-86. [Crossref] [PubMed]
- Zheng F, Meng Q, Zhang L, et al. Prognostic roles of hematological indicators for the efficacy and prognosis of immune checkpoint inhibitors in patients with advanced tumors: a retrospective cohort study. World J Surg Oncol 2023;21:198. [Crossref] [PubMed]
- Zhou Y, Fu B, Zheng X, et al. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci Rev 2020;7:998-1002. [Crossref] [PubMed]
- Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 2020;20:355-62. [Crossref] [PubMed]
- Nooh HA, Abdellateif MS, Refaat L, et al. The role of inflammatory indices in the outcome of COVID-19 cancer patients. Med Oncol 2021;39:6. [Crossref] [PubMed]
Cite this article as: Lee WJ, Dai KY, Huang WH, Su CW, Li CJ, Chen YJ. Decline of circulating monocyte and neutrophil counts in COVID-19-infected patients with cancer receiving radiotherapy. Ther Radiol Oncol 2023;7:17.


