
Treatment outcome and prognostic factors analysis of carcinoma ex pleomorphic adenoma of major salivary glands
Highlight box
Key findings
• This study identified three potential prognostic factors for carcinoma ex pleomorphic adenoma (CXPA) arising from major salivary glands—tumor origin, clinical N-stage, and pathological invasiveness subtype.
What is known and what is new?
• CXPA is an uncommon malignant tumor with aggressive behavior but the treatment outcomes and prognostic factors are rarely reported. We investigated the clinical presentation and treatment results for 22 CXPA cases in our hospital.
What is the implication, and what should change now?
• Patients with those high-risk clinical characteristics should receive more aggressive treatments.
Introduction
Carcinoma ex pleomorphic adenoma (CXPA) is an uncommon malignant tumor with aggressive behavior. The prevalence of CXPA ranges from approximately 3% to 15% among all malignant salivary gland tumors (1-4). Histologically, CXPA is a carcinoma arising from a primary or recurrent benign pleomorphic adenoma (5). Preoperative diagnosis is challenging, because the residual mixed tumor component may be quite small, and various carcinoma subtypes may be present (6). CXPA with previously treated pleomorphic adenoma was seen in 21% to 25% (1,7). Clinically, it is found frequently arising from the parotid gland, predominantly in the sixth to eighth decades of life and slightly more common in females (5).
The primary treatment of CXPA is surgery, followed by adjuvant radiotherapy for patients with poor prognostic factors (5). The role of chemotherapy is uncertain. The treatment outcomes for CXPA are unsatisfactory, with reported 5-year overall survival (OS) rates between 30% and 76% (1,2,6,8,9). The purpose of this study was to analyze the treatment outcomes and prognostic factors in patients with CXPA arising from the major salivary glands.
Methods
Patients
Inclusion criteria for this retrospective study were (I) pathologically proven CXPA arising from major salivary glands; (II) no distant metastasis at diagnosis; (III) available chart records and image data; (IV) received regular post-treatment follow-up. This study was approved by the Institutional Review Board of Changhua Christian Hospital (No. 210422), and the need for a written informed consent was waived. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). From April 2008 to May 2021, 22 eligible patients were enrolled. We reviewed hospital charts, diagnostic imaging studies, operation notes, pathological reports, and radiotherapy/chemotherapy records. There were 14 men and 8 women with a median age of 48.5 years (range, 24–84 years). Sixteen (72.7%) patients arose from the parotid gland and 6 (27.3%) from the submandibular gland. The median duration of symptom onset was about 1 year. Six (27.3%) of our patients had a prior history of benign salivary gland disease. Table 1 summarizes the patients’ characteristics. Clinical staging was defined according to the 8th edition of the American Joint Committee on Cancer (AJCC) staging system of major salivary glands. Twelve (54.5%) patients presented with early T-stage (T1 or T2), and 10 (45.5%) advanced T-stage (T3 or T4). Most patients (63.6%) had no enlargement of the regional lymph nodes, while 8 (36.4%) patients had clinical positive regional lymph node metastasis. The overall clinical stage distribution was stage I 9.1% (2/22), stage II 45.5% (10/22), stage III 27.3% (6/22), and stage IV 18.2% (4/22), respectively.
Table 1
| Characteristics | No. of case (%) |
|---|---|
| Age (year), median [range] | 48.5 [24–84] |
| Symptom duration (month), median [range] | 12.0 [0.7–240] |
| Gender | |
| Male | 14 (63.6) |
| Female | 8 (36.4) |
| Alcohol consumption | |
| Yes | 5 (22.7) |
| No | 17 (77.3) |
| Smoking | |
| Yes | 11 (50.0) |
| No | 11 (50.0) |
| Betel-nut chewing | |
| Yes | 3 (13.6) |
| No | 19 (86.4) |
| Prior history of benign salivary gland tumor | |
| Yes | 6 (27.3) |
| No | 16 (72.7) |
| Tumor origin | |
| Parotid gland | 16 (72.7) |
| Submandibular gland | 6 (27.3) |
| Clinical T-stage | |
| T1 or T2 | 12 (54.5) |
| T3 or T4 | 10 (45.5) |
| Clinical N-stage | |
| N0 | 14 (63.6) |
| N+ | 8 (36.4) |
| Primary tumor border (image) | |
| Well-defined | 12 (54.5) |
| Ill-defined | 10 (45.5) |
| Primary tumor central necrosis (image) | |
| Yes | 16 (72.7) |
| No | 6 (27.3) |
Treatment
The treatment modality consisted of surgery alone (n=2), surgery plus adjuvant radiotherapy (n=13) or chemoradiotherapy (n=5), and definitive chemoradiotherapy (n=2). Among 20 patients who received surgery, 8 patients (40%) underwent primary tumor resection and regional lymph node dissection and 12 received primary tumor excision only. For patients who received radiotherapy (n=20), the median dose of radiotherapy was 66 Gy (range, 59.4–73.5 Gy) in 33 fractions (range, 30–40 fractions), with median elapsed days of 46.5 days (range, 39–67 days). After primary treatment, 21 (95.45%) of 22 patients achieved complete remission.
Statistical analysis
The endpoints of this study were treatment outcomes and prognostic factors analyses. We used the Kaplan-Meier method to estimate OS, progression-free survival (PFS), locoregional-free survival (LRFS), and distant metastasis-free survival (DMFS). The OS was calculated from the first day of curative treatment until the date of death or last follow-up visit. The PFS was calculated from the first day of curative treatment until the first date of disease progression, death or last follow-up visit. The LRFS was measured from the first day of curative treatment until the date of local, regional, both failures or last follow-up visit. The DMFS was measured from the first day of curative treatment until the date of distant metastasis or last follow-up visit. Comparisons of various survival curves were performed using the log-rank test. The univariate Cox proportional hazards model was used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs). Analyzed factors included age, gender, alcohol consumption, smoking, betel-nut chewing, symptom duration, prior history of benign salivary gland tumor, tumor origin, clinical T-stage, clinical N-stage, and image findings (primary tumor border and central necrosis) for all 22 patients. For 20 patients who received surgery, we also analyzed additional pathological features including invasiveness subtype, resection margin, lymphovascular invasion, perineural invasion, extranodal extension. Invasiveness subtype was divided into three categories based on the presence and extent of invasion of the carcinomatous component outside the fibrous capsule, as non-invasive, minimally invasive and invasive subtype. The carcinoma component is confined within the fibrous capsule of the pleomorphic adenoma in non-invasive subtype CXPA. Minimally invasive subtype indicates malignant component of CXPA with <1.5 mm penetration into extracapsular tissue. If the malignant component extends greater than 1.5 mm outside the tumor capsule into adjacent tissue, it is classified as invasive subtype (1). All statistical analyses were performed using SPSS Statistics 27.0 (IBM Corp., Armonk, NY, USA). P value of less than 0.05 was considered statistically significant.
Results
Treatment outcome
After a median follow-up of 46.5 months (range, 13–128 months), we observed 8 relapses and 5 deaths. Of 6 relapse diseases who received operation, there were 4 invasive subtype, 1 minimally invasive subtype, and 1 non-invasive subtype. Two people didn’t undergo operation thus the status of invasiveness was unknown. The treatment failure pattern showed 4 distant metastases alone, and 4 combined distant metastases with locoregional recurrence. The median time to develop distant metastasis was 18.5 months (range, 8–63 months), and locoregional recurrence 15.5 months (range, 4–36 months). At the time of this writing, 5 patients had died and all due to uncontrolled tumors. The 5-year OS, PFS, LRFS and DMFS of all 22 patients were 71.9%, 65.3%, 78.1%, and 61.4%, respectively.
Prognostic factors analyses for all 22 patients
Kaplan-Meier survival curve analyses reveal that tumor origin is the only significant factor in predicting OS. Tumors arising from submandibular gland have a significantly worse OS (5-year rate, 26.7% vs. 88.9%, P=0.001) compared with those of parotid gland (Figure 1A). Clinical N-stage (positive vs. negative) affects OS (P=0.103, Figure 1B) but does not reach statistically significant. We combine both factors and re-analyze the data. Patients with submandibular origin and clinical positive regional nodes not only have significantly worse OS (P<0.001, Figure 2A), but also PFS (P<0.001, Figure 2B), LRFS (P=0.002, Figure 2C), and DMFS (P<0.001, Figure 2D).

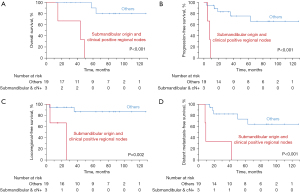
Table 2 shows the results of Cox univariate analyses for OS, PFS, LRFS and DMFS. We found that tumor origin and clinical nodal status were two potential factors in predicting survivals. Tumor arising from the submandibular (vs. parotid) gland had significantly worse OS (HR =7.79, 95% CI: 1.25–48.55, P=0.028). Patients presented with clinically regional lymph nodes positive (vs. negative) predict worse PFS (P=0.055), LRFS (P=0.081) and DMFS (P=0.064). By grouping these two factors together, we observed that patients with submandibular origin and clinical positive regional nodes had significantly worse PFS (P=0.006), LRFS (P=0.023), and DMFS (P=0.003). The calculated HR, 95% CI and P value for OS cannot be reliably estimated due to a low incidence of one subgroup. We do not do multivariate analysis due to small sample size. In addition, most variables fail to show significant results by univariate analysis.
Table 2
| Characteristics | Overall survival | Progression-free survival | Locoregional-free survival | Distant metastasis-free survival | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | ||||
| Age | |||||||||||||||
| >50 vs. ≤50 years | 2.47 | 0.39 to 15.62 | 0.337 | 1.95 | 0.47 to 8.02 | 0.357 | 5.66 | 0.58 to 55.50 | 0.137 | 2.09 | 0.50 to 8.79 | 0.314 | |||
| Symptom duration | |||||||||||||||
| >1 vs. ≤1 year | 0.69 | 0.11 to 4.16 | 0.682 | 0.59 | 0.15 to 2.39 | 0.460 | 0.20 | 0.02 to 1.92 | 0.162 | 0.46 | 0.12 to 1.88 | 0.282 | |||
| Gender | |||||||||||||||
| Male vs. female | 0.82 | 0.14 to 4.93 | 0.828 | 1.36 | 0.34 to 5.51 | 0.668 | 1.61 | 0.23 to 11.41 | 0.636 | 1.30 | 0.32 to 5.28 | 0.716 | |||
| Alcohol consumption | |||||||||||||||
| Yes vs. no | 1.82 | 0.19 to 17.06 | 0.602 | 1.12 | 0.22 to 5.59 | 0.890 | 0.03 | 0 to 847.15 | 0.511 | 1.64 | 0.32 to 8.28 | 0.552 | |||
| Smoking | |||||||||||||||
| Yes vs. no | 1.79 | 0.30 to 10.77 | 0.525 | 0.50 | 0.12 to 2.12 | 0.347 | 0.31 | 0.03 to 2.94 | 0.304 | 0.72 | 0.17 to 3.08 | 0.660 | |||
| Betel-nut chewing | |||||||||||||||
| Yes vs. no | 8.49 | 0.51 to 141.44 | 0.136 | 1.02 | 0.12 to 8.48 | 0.987 | 0.04 | 0 to 20,089.71 | 0.632 | 1.47 | 0.17 to 12.66 | 0.724 | |||
| Prior history of benign salivary gland tumor | |||||||||||||||
| Yes vs. no | 0.63 | 0.07 to 5.64 | 0.677 | 0.68 | 0.14 to 3.38 | 0.635 | 0.03 | 0 to 381.34 | 0.469 | 0.77 | 0.16 to 3.84 | 0.752 | |||
| Tumor origin | |||||||||||||||
| Submandibular vs. parotid gland | 7.79 | 1.25 to 48.55 | 0.028 | 2.54 | 0.58 to 11.03 | 0.215 | 4.74 | 0.61 to 37.11 | 0.138 | 2.77 | 0.65 to 11.82 | 0.169 | |||
| Clinical T-stage | |||||||||||||||
| T1–2 vs. T3–4 | 0.39 | 0.04 to 3.51 | 0.401 | 1.21 | 0.30 to 4.89 | 0.791 | 0.47 | 0.05 to 4.55 | 0.515 | 1.44 | 0.36 to 5.85 | 0.610 | |||
| Clinical N-stage | |||||||||||||||
| N+ vs. N0 | 4.05 | 0.66 to 24.78 | 0.130 | 4.11 | 0.97 to 17.39 | 0.055 | 7.78 | 0.78 to 78.01 | 0.081 | 3.90 | 0.92 to 16.47 | 0.064 | |||
| Primary tumor border | |||||||||||||||
| Ill-defined vs. well-defined | 1.86 | 0.31 to 11.14 | 0.499 | 2.15 | 0.51 to 9.09 | 0.297 | 4.60 | 0.48 to 44.63 | 0.188 | 2.03 | 0.48 to 8.50 | 0.333 | |||
| Primary tumor central necrosis | |||||||||||||||
| Yes vs. no | 35.49 | 0.01 to 112,720.97 | 0.386 | 2.87 | 0.35 to 23.51 | 0.325 | 38.48 | 0.01 to 265,335.99 | 0.418 | 3.30 | 0.40 to 26.87 | 0.265 | |||
HR, hazard ratio; CI, confidence interval.
Prognostic impacts of pathological features for 20 patients who received surgery
Among 20 patients who received surgical resection, 7 (35%) patients were non-invasive CXPA, 6 (30%) patients were minimally invasive CXPA, and 7 (35%) patients were invasive CXPA. Other unfavorable pathological features and its percentage revealed unsafe (involved/close) margin in 50% (10/20), perineural invasion in 25% (5/20), lymphovascular invasion in 15% (3/20), and extranodal extension in 10% (2/20) patients.
Kaplan-Meier survival curve analyses revealed that the invasiveness subtype was a significant predictor for PFS (P=0.026) and DMFS (P=0.046). We also observed a lower OS (P=0.132) and LRFS (P=0.142) for patients with invasive subtype, but the difference does not reach a statistically significant level.
Table 3 illustrated Cox univariate analyses using five pathological features (invasiveness subtype, resection margin, perineural invasion, lymphovascular invasion, and extranodal extension) for 20 patients who received surgery. We found that tumors with invasive subtype (vs. minimally invasive/non-invasive subtypes) was the only significant predictor for PFS (P=0.048). Worse but non-significant OS (P=0.158), LRFS (P=0.185), and DMFS (P=0.071) were observed for patients with invasive subtype.
Table 3
| Characteristics | Overall survival | Progression-free survival | Locoregional-free survival | Distant metastasis-free survival | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | ||||
| Invasiveness subgroup | |||||||||||||||
| Invasive vs. others | 3.66 | 0.60 to 22.24 | 0.158 | 5.63 | 1.02 to 31.15 | 0.048 | 5.24 | 0.45 to 60.52 | 0.185 | 4.80 | 0.88 to 26.31 | 0.071 | |||
| Resection margin | |||||||||||||||
| Involved/close vs. free | 2.27 | 0.38 to 13.69 | 0.371 | 2.58 | 0.47 to 14.36 | 0.278 | 2.79 | 0.25 to 31.16 | 0.406 | 2.85 | 0.52 to 15.72 | 0.229 | |||
| Lymphovascular invasion | |||||||||||||||
| Yes vs. no | 1.40 | 0.16 to 12.63 | 0.766 | 1.23 | 0.14 to 10.56 | 0.851 | 2.98 | 0.26 to 34.01 | 0.381 | 1.44 | 0.17 to 12.39 | 0.738 | |||
| Perineural invasion | |||||||||||||||
| Yes vs. no | 2.25 | 0.37 to 13.84 | 0.381 | 3.02 | 0.61 to 15.00 | 0.176 | 6.11 | 0.54 to 69.37 | 0.144 | 3.47 | 0.70 to 18.26 | 0.128 | |||
| Extranodal extension | |||||||||||||||
| Yes vs. no | 4.71 | 0.43 to 52.12 | 0.206 | 2.76 | 0.30 to 25.00 | 0.367 | 7.65 | 0.47 to 123.41 | 0.152 | 4.52 | 0.46 to 44.19 | 0.194 | |||
HR, hazard ratio; CI, confidence interval.
Discussion
CXPA is an uncommon malignancy among head and neck region and reports for treatment outcome of CXPA are relatively rare. It is important for patients diagnosed as CXPA treated by a multidisciplinary team. Patients are diagnosed and staged mostly by head and neck surgeons, and they may undergo operation or definitive chemoradiotherapy depending on the disease status and patients’ preference. A good efficacy and functional impact balance must be evaluated and well-explained to the patient (10). After operation, radiation oncologists decide the radiotherapy treatment planning according to the surgical finding and pathologic features reported by the pathologist. Post-operative adjuvant radiotherapy is performed within 6 weeks after surgery when indicated. In locally advanced or metastatic disease, hematology oncologists provide best medication choices for these patients. There may also be a role in stereotactic body radiation therapy (SBRT) for oligometastatic patients (11).
Severe retrospective studies with limited case numbers revealed 5-year rates of 30–76% for OS (1,2,6,8,9) and 37–75% for disease-specific survival (DSS) (1,2,6-9,12). A multi-institutional retrospective study in the northern Japan area with shorter follow-up time reported a 3-year OS 79.9% and PFS 76.8% for 33 patients with CXPA of the parotid gland (13). By using the Surveillance, Epidemiology, and End Results (SEER) database, Gupta et al. identified 619 patients of major salivary gland CXPA from 1973 to 2015 and found the 5-year OS of 68.5% and DSS of 80.4% (14). Our results of 5-year OS 71.9% and PFS 65.3% are compatible with the literature.
The reported overall treatment failure rates for CXPA were 33.3–53.0% (6,8,13). Eight of 22 (36.4%) our patients encountered treatment failure. Regarding detailed treatment failure pattern, only a few reports showed the data. In our study, distant metastases account for 36.4% of the patients and outnumber locoregional recurrence rate (18.2%). There were 6 patients in this study presented with early stage (stage I or II) while 16 patients presented with advanced stage (stage III or IV). It may be the reason that distant relapse rate was two-fold compared to locoregional recurrence rate. Two prior studies showed similar failure patterns- more distant failures rather than locoregional recurrences (13,15). Hu et al. from Shanghai, China reported 54 distant metastases (16.2%) and 30 locoregional recurrences (8.9%) among 334 patients who had available follow-up information (15). They defined a subgroup of 174 patients with widely invasive CXPA and observed 53 distant failures (30.6%) and 29 locoregional recurrences (16.8%) (15). There were 8 distant failures (24.2%) and 5 locoregional recurrences (15.2%) among 33 patients in Suzuki et al.’s study from Japan (13). In contrast, another two studies found more locoregional recurrences rather than distant metastasis (8,12). Zhao et al. studied 51 patients in Zhejiang Cancer Hospital, China, and revealed a 39.2% locoregional recurrent rate and a 27.5% distant metastasis rate of their patients (8). Ye et al. investigated 135 patients with frankly invasive CXPA from Beijing, China and showed more than half these cases (73 of 135; 54.1%) developed local recurrences; 25 (18.5%) developed regional metastasis; 21 (15.6%) developed distant metastases (12). In addition, Chen et al. collected 63 patients of parotid CXPA from the University of California, San Francisco (UCSF) and reported 20 local recurrences (31.7%), 8 regional recurrences (12.7%), and 27 distant metastases (42.9%) (9). Based on these data, it is still non-conclusive regarding the most frequent site of treatment failure for CXPA.
Many predicting factors of DSS were reported for CXPA. Hu et al. reviewed a largest sample size of 361 CXPA patients from a single institute (15). Among them, 334 patients had available follow-up information. They found that age (P<0.001), T-stage (P<0.001), N-stage (P<0.001), invasiveness (P<0.001), histologic grade (P<0.001), proportion of carcinoma (P<0.001), perineural invasion (P<0.001), and vascular invasion (P=0.010) were significant predictors for DSS by Kaplan-Meier analysis (15). Cox univariate analysis revealed the same results (15). In Cox multivariate analysis, T-stage (P=0.002), N-stage (P<0.001) and invasiveness (P=0.002) were significant predictors for DSS (15). A total of 151 patients with CXPA were reviewed by Ye et al. from Beijing, China and revealed that T-stage, N-stage, overall clinical stage, invasiveness, and malignant subtype were significant risk factors for DSS (12). Two reports from the SEER database, showed several prognostic factors. Gupta et al. enrolled 619 patients of major salivary gland CXPA from 1973 to 2015 and found that high grade, late stage, larger tumor size (≥4 cm), extra-parenchymal extension, multiple positive regional nodes, and initial distant metastasis were poor predictors for DSS by univariate analysis (14). Among these factors, a tumor size ≥4 cm, multiple positive lymph nodes and initial distant metastasis were independent prognostic factors using multivariable analysis (14). Chen et al. extracted 278 patients with CXPA arising from parotid gland in the SEER databank [1988–2009] and revealed race, multiple metastatic lymph nodes and initial distant metastasis were independent prognostic factors of DSS (16). Katabi et al. reviewed 43 patients with CXPA from the Memorial Sloan-Kettering Cancer Center and illustrated that vascular invasion and initial distant metastases conferred significant worse DSS (P<0.05) (17). Olsen et al. investigated 66 patients with CXPA who received primary treatment at Mayo Clinic and found that clinical adenopathy, overall clinical stage, and local extension beyond the gland evaluated by clinical exam were significant predictors for DSS by univariate analysis (6). In a detailed pathologic study from the same institute on the same patients, Lewis et al. reported that pathologic T-stage, pathologic N-stage, overall pathologic stage, tumor size, histologic grade, proportion of carcinoma, extent of invasion, and proliferation index of the carcinoma component affected DSS significantly by univariate analysis (18).
Predictors for OS were less frequently reported. In Mayo Clinic’s study mentioned above, factors predicting DSS also affected OS, including clinical factors (6) and pathologic factors (18). In UCSF’s report, T-stage, N-stage, facial nerve involvement, and the use of postoperative radiation therapy were identified as significant predictors for OS using univariate analysis but only pathologic lymph node metastasis was the independent predictor by multivariate analysis (9). In Zhao et al.’s study, factors significantly associated with OS were age, histological grade, invasiveness, T-stage, lymph node involvement, overall clinical stage and perineural invasion (8). Using Cox multivariate analysis, T-stage, lymph node involvement, histological grade and perineural invasion were identified as independent prognostic factors for OS (8).
In our study, Kaplan-Meier survival analyses revealed that tumor origin affected OS significantly (P=0.001) and clinical N-stage had a potential impact on OS (P=0.103). In addition, patients with submandibular origin plus clinical positive regional lymph nodes had significantly worse OS (P<0.001), as well as for PFS (P<0.001), LRFS (P=0.002), and DMFS (P<0.001). Among 20 patients who received surgery, invasiveness (invasive vs. minimally invasive/non-invasive subtypes) was a significant predictor for PFS (P=0.026) and DMFS (P=0.046), a lower but non-significant factor in predicting OS (P=0.132) and LRFS (P=0.142).
All patients in this study received novel radiotherapy techniques, with 3D conformal radiotherapy (3D-CRT), intensity modulated radiation therapy (IMRT), image-guided radiotherapy (IGRT) or tomotherapy. The novel radiation technique decreased radiation doses of the organs at risk while preserving locoregional control. Limitations of this study include small patient’s number, non-uniform treatment, and retrospective nature, etc.
Recently the application of artificial intelligence has progressed in the clinical medicine field (19). De Felice et al. applied machine learning approaches to make decision tree algorithms based on their clinical data to analyze survival outcomes and predict recurrence rate in patients with high-risk salivary gland malignant tumors (19). On the other hand, there were some studies involving the relationship between oral microbiome and cancer oncogenesis. Identifying a microbiome signature may potentially define different classes to predict cancer risk, treatment outcomes and even RT-related oral mucositis based on individual radiosensitivity (20). These novel techniques may help in clinical decision making and prediction of survivals.
Conclusions
Based on our results and above discussion, we conclude that treatment outcomes for CXPA still have much room for improvement (a 33.3–53.0% overall treatment failure rate; 5-year rates of OS, 30–76% and DSS, 37–75%). Most patients fail distantly and how to strengthen the systemic therapy is an urgent need in the future. The most important factors in predicting survivals are invasiveness and N-stage, followed by overall stage, T-stage, and histologic grade. Patients with these poor prognostic factors may need the application of postoperative radiotherapy. Other minor factors such as margin status, perineural invasion and vascular invasion may also be taken into consideration. In addition, accurate diagnosis and aggressive surgical and radiological management of patients presenting with CXPA may increase the survival rates. Due to the small patient number in this study, prospective data or larger number retrospective study is needed to confirm this conclusion.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tro.amegroups.com/article/view/10.21037/tro-23-11/coif). J.C.L. serves as an unpaid editorial board member of Therapeutic Radiology and Oncology from May 2022 to April 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Changhua Christian Hospital (No. 210422), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zbären P, Zbären S, Caversaccio MD, et al. Carcinoma ex pleomorphic adenoma: diagnostic difficulty and outcome. Otolaryngol Head Neck Surg 2008;138:601-5. [Crossref] [PubMed]
- Lüers JC, Wittekindt C, Streppel M, et al. Carcinoma ex pleomorphic adenoma of the parotid gland. Study and implications for diagnostics and therapy. Acta Oncol 2009;48:132-6. [Crossref] [PubMed]
- Gnepp DR. Malignant mixed tumors of the salivary glands: a review. Pathol Annu 1993;28:279-328. [PubMed]
- Spiro RH, Huvos AG, Strong EW. Malignant mixed tumor of salivary origin: a clinicopathologic study of 146 cases. Cancer 1977;39:388-96. [Crossref] [PubMed]
- Antony J, Gopalan V, Smith RA, et al. Carcinoma ex pleomorphic adenoma: a comprehensive review of clinical, pathological and molecular data. Head Neck Pathol 2012;6:1-9. [Crossref] [PubMed]
- Olsen KD, Lewis JE. Carcinoma ex pleomorphic adenoma: a clinicopathologic review. Head Neck 2001;23:705-12. [Crossref] [PubMed]
- Nouraei SA, Hope KL, Kelly CG, et al. Carcinoma ex benign pleomorphic adenoma of the parotid gland. Plast Reconstr Surg 2005;116:1206-13. [Crossref] [PubMed]
- Zhao J, Wang J, Yu C, et al. Prognostic factors affecting the clinical outcome of carcinoma ex pleomorphic adenoma in the major salivary gland. World J Surg Oncol 2013;11:180. [Crossref] [PubMed]
- Chen AM, Garcia J, Bucci MK, et al. The role of postoperative radiation therapy in carcinoma ex pleomorphic adenoma of the parotid gland. Int J Radiat Oncol Biol Phys 2007;67:138-43. [Crossref] [PubMed]
- De Felice F, de Vincentiis M, Valentini V, et al. Management of salivary gland malignant tumor: the Policlinico Umberto I, "Sapienza" University of Rome Head and Neck Unit clinical recommendations. Crit Rev Oncol Hematol 2017;120:93-7. [Crossref] [PubMed]
- Franzese C, Ingargiola R, Tomatis S, et al. Metastatic salivary gland carcinoma: A role for stereotactic body radiation therapy? A study of AIRO-Head and Neck working group. Oral Dis 2022;28:345-51. [Crossref] [PubMed]
- Ye P, Gao Y, Mao C, et al. Carcinoma Ex Pleomorphic Adenoma: Is It a High-Grade Malignancy? J Oral Maxillofac Surg 2016;74:2093-104. [Crossref] [PubMed]
- Suzuki M, Matsuzuka T, Saijo S, et al. Carcinoma ex pleomorphic adenoma of the parotid gland: a multi-institutional retrospective analysis in the Northern Japan Head and Neck Cancer Society. Acta Otolaryngol 2016;136:1154-8. [Crossref] [PubMed]
- Gupta A, Koochakzadeh S, Neskey DM, et al. Carcinoma ex pleomorphic adenoma: A review of incidence, demographics, risk factors, and survival. Am J Otolaryngol 2019;40:102279. [Crossref] [PubMed]
- Hu YH, Zhang CY, Xia RH, et al. Prognostic factors of carcinoma ex pleomorphic adenoma of the salivary glands, with emphasis on the widely invasive carcinoma: a clinicopathologic analysis of 361 cases in a Chinese population. Oral Surg Oral Med Oral Pathol Oral Radiol 2016;122:598-608. [Crossref] [PubMed]
- Chen MM, Roman SA, Sosa JA, et al. Predictors of survival in carcinoma ex pleomorphic adenoma. Head Neck 2014;36:1324-8. [Crossref] [PubMed]
- Katabi N, Gomez D, Klimstra DS, et al. Prognostic factors of recurrence in salivary carcinoma ex pleomorphic adenoma, with emphasis on the carcinoma histologic subtype: a clinicopathologic study of 43 cases. Hum Pathol 2010;41:927-34. [Crossref] [PubMed]
- Lewis JE, Olsen KD, Sebo TJ. Carcinoma ex pleomorphic adenoma: pathologic analysis of 73 cases. Hum Pathol 2001;32:596-604. [Crossref] [PubMed]
- De Felice F, Valentini V, De Vincentiis M, et al. Prediction of Recurrence by Machine Learning in Salivary Gland Cancer Patients After Adjuvant (Chemo)Radiotherapy. In Vivo 2021;35:3355-60. [Crossref] [PubMed]
- Orlandi E, Iacovelli NA, Tombolini V, et al. Potential role of microbiome in oncogenesis, outcome prediction and therapeutic targeting for head and neck cancer. Oral Oncol 2019;99:104453. [Crossref] [PubMed]
Cite this article as: Chang YH, Kuo C, Chang TH, Chen MK, Lin JC. Treatment outcome and prognostic factors analysis of carcinoma ex pleomorphic adenoma of major salivary glands. Ther Radiol Oncol 2023;7:14.


