
Molecular subtypes and clinicopathological features of breast cancer in Libya: a first glance
Highlight box
Key findings
• Luminal B tumors are the most common molecular subtype and higher prevalence compared to triple-negative breast cancer (TNBC) in Libya.
• TNBC, which is the less common type, is a more aggressive subtype of breast cancer.
What is known and what is new?
• Race and geographic distribution of molecular subtypes are markedly different.
• First descriptive study of molecular subtype of breast cancer in Libya.
What is the implication, and what should change now?
• Libyan patients have an aggressive phenotype compared to their North African counterparts.
• Additional prospective studies documenting survival rates of the different molecular subtypes are needed to correlate with the clinical course.
Introduction
Breast cancer (BC) is one of the most common cancers and the fifth leading cause of cancer death. It is predicted to have surpassed lung cancer as the most frequent cancer among women worldwide. According to GLOBOCAN 2020 data, BC affects 2.3 million people worldwide each year, resulting in 684,996 deaths (1). Despite having similar histological characteristics, BC is a widely heterogeneous malignancy in terms of clinical presentation, morphology, molecular markers, prognosis, and treatment response, with ethnicity playing a substantial part in this diversity (2). Histological grade, tumor size, lymph node status, and receptor status are all known to impact disease prognosis and treatment response and are thus powerful prognostic and predictive indicators.
Immunohistochemistry (IHC), or the more precise technique of microarray-based gene expression profiling (GEP), is used to classify BC subtypes. In resource-limited environments, IHC staining is an excellent substitute for GEP. Perou et al. (3) identified four molecular subtypes based on microarray gene expression in 2000: luminal, human epidermal growth factor receptor 2 (HER2)-enriched, basal-like, and normal basal-like (3). Further research enabled the luminal group to be divided into two subgroups (luminal A and B) (4,5).
Studies show that molecular subtypes are distinctly variable in terms of race and geographic distribution (6,7). This molecular diversity is etiologically and therapeutically significant since it maps disparities in therapy efficiency and prognosis (8,9). Moreover, ethnic disparities in BC outcomes, particularly among younger women, may be partly explained by the unequal distribution of molecular subtypes (10,11).
Understanding the regional distribution of BC subtypes is relevant for several reasons. If the distribution of receptor status is different from other neighboring parts, factors contributing to this variation would need to be investigated thoroughly, and if one subtype predominates, the introduction of IHC subtypes in areas where it is not available would need to be ascertained.
In Africa, the distribution of BC subtypes is debatable. Some studies demonstrate that the majority of cases have low hormone receptor expression (12), but a recent comprehensive review and meta-analysis results indicate that the majority of cases have high hormone receptor expression (13). In Libya, cancer epidemiology studies are very limited and are generally considered insufficient to accurately reflect the disease status. However, several local studies consistently reported BC as the most common cancer with a frequency of around 20% (14,15). Zarmouh et al. (15) reported that Libyan females when diagnosed with BC, despite presenting at a relatively younger age, usually present at an advanced stage. Gusbi et al. (16) estimated that more than half of Libyan females with BC are present in stages III/IV. Additionally, the local distribution of BC molecular subtypes has not been thoroughly investigated and, as such, constitutes a knowledge gap in understanding the nature of BC cases in Libya.
This work aims to document the clinicopathological features and molecular subtypes of invasive BC cases from Libya. In addition to examine the association between clinicopathological features and molecular BC subtypes.
Methods
This is a retrospective observational descriptive study. All Libyan female BC patients treated and monitored at the radiotherapy department in Tripoli University Hospital from January 2009 to December 2012 with pathology proven BC were retrospectively included. Data were retrieved directly from patient records through the Tripoli University Hospital registry and archive following attainment of the ethics board approval.
Variables of interest include demographic characteristics, medical history, clinical staging, complete histopathological reporting, and treatment received. Tumor-node-metastasis (TNM) staging was to be used according to the American Joint Committee on Cancer. The tumor grade was classified according to the Scarff Bloom and Richardson histological system.
For the immunoperoxidase experiment, paraffin blocks of breast mass were typically stained with IHC instrument. The sections were stained using the Ventana Benchmark® GX in automatic mode (Ventana Medical Systems Inc., Tucson, AZ, USA). The tissue tumors which were 4-µm thick, underwent xylene cleaning, ethanol rehydration, and distilled water rinsing. After that, the slides were treated with primary antibodies for 30 min at room temperature, followed by a 10-min incubation with hydrogen peroxide, a chromogen (diaminobenzidine), and hematoxylin as a counterstain. After then, the slides were dehydrated. The antibody clones were monoclonal, developed in rats, consisted of SP1 for the estrogen receptor (ER) and 1E2 for the progesterone receptor (PR), and HER2/neu (4B5) manufactured by Ventana Medical Systems, Inc. In less than 5% of the neoplastic cells, the presence or lack of hormone receptors was determined solely by nuclear reactivity. Weak incomplete membrane staining was used to determine the HER2 status of tumors scoring 0 or 1+ whereas strong complete membrane staining was used to determine the HER2 status of tumors scoring 3+. Colorimetric in situ hybridization (CISH) was used to reevaluate cases of borderline HER2 with a score of 2+.
Tumor receptor status was documented and subsequently divided into subtypes as follows: luminal A (ER+, PR+/PR−, HER2− score 0), luminal B (ER+, PR+/PR−, HER2+), HER2-enriched (ER−, PR−, HER2+), and triple-negative breast cancer (TNBC) (ER−, PR−, HER2−). HER2+ cases include HER2 score 1+, and equivocal score 2 cases with negative CISH. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Bioethics Committee at Biotechnology Research Center (BEC-BTRC), Tripoli, Libya (Ref. No. NBC:001.H.23.11) and informed consent was taken from all the patients.
Statistical analysis
Using the statistical package for the social sciences (SPSS) version 24 (IBM Corp, Armonk, NY, USA), baseline demographic characteristics, and tumor-related variables were analyzed. The Chi-square test was used to assess the distribution of these clinicopathological features among BC subtypes.
Results
A total of 319 cases were examined. The mean age was 47.3 (±10.7) years. Invasive ductal carcinoma (IDC) was the most prevalent histological type (80.3%), and the majority of patients were premenopausal (59.2%) as shown in Table 1.
Table 1
| Variables | Subcategories | Frequency | Percentage (%) |
|---|---|---|---|
| Age (years) | ≤35 | 47 | 14.7 |
| 36–45 | 106 | 33.2 | |
| 46–55 | 101 | 31.7 | |
| 56–65 | 45 | 14.1 | |
| ≥66 | 20 | 6.3 | |
| Size (cm) | <2 | 48 | 15.0 |
| 2–5 | 191 | 59.9 | |
| >5 | 75 | 23.5 | |
| Unknown | 5 | 1.6 | |
| Menopausal status | Premenopausal | 189 | 59.2 |
| Perimenopausal | 10 | 3.1 | |
| Postmenopausal | 117 | 36.7 | |
| Unknown | 3 | 0.9 | |
| Family history | Yes | 63 | 19.7 |
| No | 233 | 73.0 | |
| Unknown | 23 | 7.2 | |
| Histological type | Ductal | 256 | 80.3 |
| Lobular | 22 | 6.9 | |
| Others | 12 | 3.8 | |
| Unknown | 29 | 9.1 | |
| Lymph node status | Positive | 211 | 66.1 |
| Negative | 95 | 29.8 | |
| Unknown | 13 | 4.1 | |
| Histological grade | Grade 1 | 21 | 6.6 |
| Grade 2 | 118 | 37.0 | |
| Grade 3 | 123 | 38.6 | |
| Unknown | 57 | 17.9 | |
| Surgery | Yes | 307 | 96.2 |
| No | 12 | 3.8 | |
| Chemotherapy | Yes | 307 | 96.2 |
| No | 8 | 2.5 | |
| Unknown | 4 | 1.3 |
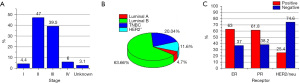
At the time of diagnosis, the average tumor size was 3.9 (±2.2) cm, and the distribution of stages II and III was 47.0% and 39.5%, respectively (Figure 1A). More than 60% of the samples were lymph node-positive. Regarding molecular subtype distribution, luminal B constituted 63.66% of all cases, whereas TNBC was 20.04%. HER2+ and luminal A were 11.6% and 4.7%, respectively (Figure 1B). ER+ rate was 63.0%, PR+ rate was 61.8%, and HER2+ rate was 25.4% (Figure 1C).
The molecular subtypes differed significantly by lymph node (P=0.017). According to histological grade, the molecular subtypes have also demonstrated a very significant difference (P=0.008). The distribution of distinct clinical and pathological traits among various molecular subtypes. The majority of luminal B cancers have tumor sizes of between 2 and 5 cm. These results, however, were not statistically significant (P=0.088). Luminal B, on the other hand, was 72% related to the metastatic lymph node (Table 2). Around 67.4% had modified radical mastectomy and 29.2% had breast-conserving therapy.
Table 2
| Variables | Subcategories | Luminal A | Luminal B | TNBC | HER2 positive | Total | P value |
|---|---|---|---|---|---|---|---|
| Age groups (years) | ≤35 | 4 | 26 | 10 | 7 | 47 | 0.517 |
| 36–45 | 2 | 70 | 24 | 10 | 106 | ||
| 46–55 | 4 | 65 | 21 | 11 | 101 | ||
| 56–65 | 2 | 30 | 7 | 6 | 45 | ||
| ≥66 | 3 | 10 | 4 | 3 | 20 | ||
| Menopausal status | Premenopausal | 8 | 122 | 38 | 21 | 189 | 0.234 |
| Perimenopausal | 0 | 5 | 3 | 2 | 10 | ||
| Postmenopausal | 7 | 73 | 25 | 12 | 117 | ||
| Unknown | 0 | 1 | 0 | 2 | 3 | ||
| Family history of breast cancer | Yes | 2 | 34 | 14 | 13 | 63 | 0.206 |
| No | 11 | 151 | 49 | 22 | 233 | ||
| Unknown | 2 | 16 | 3 | 2 | 23 | ||
| Histological type of breast cancer | Ductal | 14 | 155 | 56 | 31 | 256 | 0.201 |
| Lobular | 0 | 19 | 1 | 2 | 22 | ||
| Others | 0 | 7 | 5 | 0 | 12 | ||
| Unknown | 1 | 20 | 4 | 4 | 29 | ||
| Histological grade of the tumor | Grade 1 | 0 | 15 | 4 | 2 | 21 | 0.008 |
| Grade 2 | 8 | 87 | 14 | 9 | 118 | ||
| Grade 3 | 6 | 61 | 37 | 19 | 123 | ||
| Unknown | 1 | 38 | 11 | 7 | 57 | ||
| Lymph node status | Positive | 12 | 145 | 32 | 22 | 211 | 0.017 |
| Negative | 3 | 49 | 29 | 14 | 95 | ||
| Unknown | 0 | 7 | 5 | 1 | 13 | ||
| T | T1 | 3 | 19 | 8 | 1 | 31 | 0.242 |
| T2 | 7 | 104 | 45 | 26 | 182 | ||
| T3 | 4 | 57 | 8 | 8 | 77 | ||
| T4 | 1 | 18 | 4 | 2 | 25 | ||
| Unknown | 0 | 3 | 1 | 0 | 4 | ||
| N | N0 | 3 | 54 | 29 | 15 | 101 | 0.014 |
| N1 | 6 | 72 | 16 | 11 | 105 | ||
| N2 | 6 | 44 | 15 | 2 | 67 | ||
| N3 | 0 | 27 | 4 | 9 | 40 | ||
| Unknown | 0 | 4 | 2 | 0 | 6 | ||
| M | M0 | 14 | 190 | 61 | 34 | 299 | 0.957 |
| M1 | 1 | 11 | 5 | 3 | 20 | ||
| Staging | 0 | 0 | 7 | 3 | 0 | 10 | 0.619 |
| I | 1 | 8 | 5 | 0 | 14 | ||
| II | 7 | 89 | 33 | 21 | 150 | ||
| III | 6 | 87 | 20 | 13 | 126 | ||
| IV | 1 | 10 | 5 | 3 | 19 | ||
| Size (cm) | <2 | 3 | 30 | 14 | 1 | 48 | 0.088 |
| 2–5 | 9 | 113 | 42 | 27 | 191 | ||
| >5 | 3 | 54 | 9 | 9 | 75 | ||
| Unknown | 0 | 3 | 1 | 1 | 5 | ||
| Primary surgery done to the patient | Mastectomy | 9 | 138 | 39 | 28 | 214 | 0.517 |
| Conserving surgery | 5 | 57 | 23 | 8 | 93 | ||
| Missing | 1 | 6 | 4 | 1 | 12 |
TNBC, triple-negative breast cancer; HER2, human epidermal growth factor receptor 2; T, primary tumor; N, regional lymph nodes; M, distant metastasis.
Discussion
Management of BC relies on the availability of robust clinical and pathological prognostic and predictive factors to guide patient decision-making and the selection of treatment options (17).
The present study performed a molecular classification of BC cases in the Libyan population to understand tumor behavior and to compare it to other ethnic populations. In North Africa, little is known about the molecular distribution of BC. To this date, no studies have reported the distribution of BC molecular subtypes in Libyan women (16).
Age at diagnosis and risk factors for BC
Age at diagnosis is an important prognostic factor. Tumors diagnosed at a younger age are generally more aggressive and/or less responsive to treatment (18). Compared to women aged 40–60 years, women younger than 40 have higher BC mortality (19). Study’s mean age at diagnosis was 47.3 (±10.7) years. This is similar to a report in 2020 in Libya (16) and other North African and Sub-Saharan countries (20-23). The results are also consistent with the mean age reported in various Arab populations and South Asian populations (24-26). But it is early compared to global figures where results from western countries and Europe report a mean age at diagnosis a decade older than Arab nations (14,27). This early age at diagnosis may be a result of the large percentage of women in Libya between the ages of 25 and 50 years, according to the distribution of the population in the census. Further research is required to identify any underlying genetic or environmental factors that may render Libyan females more prone to developing the disease at a younger age.
Data from GLOBOCAN 2018 estimated that 5–10% of new cases have a family history (FH) of breast or ovarian cancers (28). However, this is only partly explained by inherited mutations and many critical factors affecting BC risks, such as breast density and body mass index (BMI), show familial clustering, confounding the impact of FH on BC incidence rates (29). Therefore, genetic counseling and testing should be offered to patients in high-risk groups as defined by the National Comprehensive Cancer Network (NCCN) guidelines. Positive FH was reported in 19.7% of samples. Those rates are lower compared to the 28.8% reported from western Libya in 2018 (16).
About 60% of patients were premenopausal (Table 1). This pattern follows that of developing countries, with 60.78% reported from Algeria (30) and 67% in the Sub-Saharan region (31), but contradicts that of developed countries (32). BC epidemiology shows distinct distribution patterns when stratified by menopausal status. The proportion of premenopausal cases and deaths in 2018 was greatest in low-income countries, whereas in higher-income countries, there was a greater burden of postmenopausal cases (33). This global diversity of disease incidence is mostly due to the variation in exposure to reproductive and lifestyle risk factors. Population aging, together with the westernization of lifestyle, will soon result in a higher prevalence of postmenopausal cases (34).
Clinicopathological features of BC in Libya
TNM staging plays a central role in predicting disease prognosis, with higher stages carrying a worse prognosis. The current results showed 47% of cases were at stage II and about 39.5% of cases were at stage III (Figure 1A). This stage distribution is consistent with Gusbi et al. with 50.4% of cases at stage II and 32.7% at stage III (16). This stage distribution is comparable to the AJCC system estimation, which highlighted stages II and III as the most common (50.4% and 23.7%, respectively) but varies significantly when compared to the Sub-Saharan region distribution, where (73–88%) of women presented at advanced stages namely III and IV (31).
Tumor size is directly related to the increased number of axillary lymph nodes involved and the risk of regional metastasis, thereby, it is considered an important prognostic factor. The mean tumor size in the present study was 3.9 (±2.1) cm. The study found no statistically significant difference between the size of tumor and the molecular subtypes. Only 15% of patients presented with tumor sizes less than 2 cm (Table 1), which raises questions regarding the cause of delayed recognition of cases in our community. These figures are similar to studies in Tunisia and Morocco (20,21) and other Arab countries (24,35) but are slightly higher when compared to Algeria’s (23).
Lymph node status continues to be one of the most powerful markers of long-term prognosis in primary BC. Following surgery, axillary lymph node dissection (ALND) is linked with a greater incidence of lymphoedema affecting the upper limbs in up to 25% of women, constituting a significant contribution to disease-related morbidity (36). And even small tumors (<2 cm) have a worse prognosis in the presence of pathologic node involvement. Lymph node-positive tumors were observed in 66% of the population. Such a high prevalence is also observed in Tripoli/Libya (37) and the majority of the developing world. This includes Arabs, central Africans, and Iran (35). However, in developed countries, the majority of lymph nodes were negative (38).
Histology type and histology tumor grade: most patients were in grade II and grade III proportions in our study population. Or regarding tumor grade, 118 patients (37.0%) were in grade II and 123 patients (38.6%) were in grade III as shown in Table 2. This is consistent with the data reported from western Libya between 2003 and 2018 (16). Similar results are also reported by Kakarala et al. (39) when Asian Indian and Pakistani women in the US were studied. However, several studies on Arab and Asian ethnicities showed a predominance of grade II tumors over grade III, with the former constituting more than 50% of cases (20,23). IDC was the predominant histological type in our study. This matches the results from the western Libya report and is in concordance with global results. Lobular carcinoma represents the second most common histological type.
Hormonal receptor and distribution of molecular subtypes in Libya
In this study, the rate of ER+ and PR+ was much higher than that of HER2. Around 63.0% of the patients were ER+ whereas 61.8% were PR+. HER2+ rate was 25.4% (Figure 1C). Positive receptor status is a recognized prognostic determinant and is estimated to reduce BC mortality by around one-third (40). A meta-analysis demonstrated that in patients with ER+ disease, tamoxifen significantly reduced the risk of recurrence by 39% and death by 30% throughout 15 years of follow-up (41). Therefore, the introduction of IHC subtypes in areas where it is not available needs to be ascertained, as they allow improved patient stratification and permit designing aggressive treatment strategies while increasing active surveillance in patients with a high risk of tumor recurrence.
Molecular subtypes: studies confirmed that molecular subtypes are distinctly variable in terms of race, geographic distribution, survival, and response to treatment, and those treatment selections are dictated by the specific molecular profile (6,7,42). Hormonal therapy, for example, is only helpful for patients with cancers that express ER and/or PR. Moreover, ethnic disparities in BC outcomes, held particularly among younger women, may be partly explained by the unequal distribution of molecular subtypes (10,11). Luminal A and B are prevalent in elderly white women and have a promising prospect (7,43). TNBCs are frequently poorly differentiated, resistant to hormone therapy, and have a poor prognosis. Hence they are typically treated with radiochemotherapy. HER2-enriched forms have a bad prognosis as well, as they are not sensitive to hormonal therapy and should be treated with HER2-targeted therapy instead (44,45).
In this study, luminal B was the most prevalent (63.66%) followed by the triple-negative type (20.04%), HER2-expressing tumors (11.6%), and luminal A (4.7%) (Figure 1B). Luminal predominance is similar to Iraq, Morocco, Brazil, and China (46-48) but differs from the reports from Egypt, Tunisia, Morocco, Algeria, and Sub-Saharan Africa where luminal A was the most common. Compared to luminal A, luminal B is much more aggressive and is characterized by poor differentiation, a high bone metastasis rate, and a worse prognosis (49). Surprisingly, luminal A was the least common in our population (4.7%) (Figure 1B). This distribution is uncommon as the majority of the world reports luminal A as the most common molecular subtype (20-22,26,27,35,49,50). TNBS was the second most common subtype among Libyan females (20%) as shown by previous studies on Africans (21,25). However, this prevalence is higher than reported in Morocco, Saudi Arabia, and Iraq (20,47).
The HER2/neu subtype in this study accounted for 11.6% of all BC cases (Figure 1B), while the HER2 receptor positivity rate was 25.4% (Figure 1C). It is higher than western and Asian proportions. Earlier studies in Arab and African countries revealed similar figures (21,47,48,51).
There are some limitations to this study that should be noted. First, its retrospective, descriptive nature, and second, the incomplete medical records lacking important demographic and risk factor-related variables. Third, the portion of BC patients included in the study are those referred to the radiotherapy department, which may explain the high proportion of advanced disease. Our classification of molecular subtypes is based on IHC profiling. Most studies have found comparable results between gene expression and IHC profiling. No data was available about Ki67 status, at that time which was not used as a routine. We also did not differentiate between triple-negative and basal-like tumors. However, it has been confirmed that the triple-negative phenotype of ER−, PR−, and HER2− is a reasonable proxy (80–90%) for basal-like BC.
Conclusions
BC has a distinct epidemiology in Libya; younger age of onset, advanced stage at diagnosis. There is a higher proportion of aggressive tumors, a larger tumor size, and a higher histopathological grade. These characteristics arise from risk factor profiles that differ from those seen in developing countries, resulting in a substantially lower incidence of this disease in postmenopausal women. The findings show luminal B tumors are the most common in Libya and also have a high prevalence of TNBC. This implies BC in Libya has an aggressive phenotype in comparison to other North African countries. Luminal A was the least common in our population compared to females from neighboring countries these features are alarming and suggest that Libyan females tend to have worse prognosis subtypes. The prevalence of BC at a younger age in Libya prioritizes the demand for a screening program to be implemented at a younger age.
Given the reported disease burden in the region and the absence of accurate figures reflecting the disease status in Libya, we highly recommend including data from other local cancer centers to study the distribution of BC subtypes. Emphasis on the need for the wider introduction of IHC staining, formal national cancer registries, and organized epidemiological surveys directed towards investigating the molecular subtypes trends over time and among varying ethnicities are crucial. More efforts should be directed toward the introduction of a national screening program for high-risk groups. Additional prospective studies documenting survival rates of the different molecular subtypes are needed to correlate with the clinical course.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tro.amegroups.com/article/view/10.21037/tro-23-1/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Bioethics Committee at Biotechnology Research Center (BEC-BTRC), Tripoli, Libya (Ref. No. NBC:001.H.23.11) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Wiencke JK. Impact of race/ethnicity on molecular pathways in human cancer. Nat Rev Cancer 2004;4:79-84. [Crossref] [PubMed]
- Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747-52. [Crossref] [PubMed]
- Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001;98:10869-74. [Crossref] [PubMed]
- Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol 2011;5:5-23. [Crossref] [PubMed]
- Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006;295:2492-502. [Crossref] [PubMed]
- Yamamoto S, Maki DD, Korn RL, et al. Radiogenomic analysis of breast cancer using MRI: a preliminary study to define the landscape. AJR Am J Roentgenol 2012;199:654-63. [Crossref] [PubMed]
- DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin 2019;69:438-51. [Crossref] [PubMed]
- Prat A, Pineda E, Adamo B, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast 2015;24:S26-35. [Crossref] [PubMed]
- Scott LC, Mobley LR, Kuo TM, et al. Update on triple-negative breast cancer disparities for the United States: A population-based study from the United States Cancer Statistics database, 2010 through 2014. Cancer 2019;125:3412-7. [Crossref] [PubMed]
- Field TS, Buist DS, Doubeni C, et al. Disparities and survival among breast cancer patients. J Natl Cancer Inst Monogr 2005;88-95. [Crossref] [PubMed]
- Bird PA, Hill AG, Houssami N. Poor hormone receptor expression in East African breast cancer: evidence of a biologically different disease? Ann Surg Oncol 2008;15:1983-8. [Crossref] [PubMed]
- Eng A, McCormack V, dos-Santos-Silva I. Receptor-defined subtypes of breast cancer in indigenous populations in Africa: a systematic review and meta-analysis. PLoS Med 2014;11:e1001720. [Crossref] [PubMed]
- Boder JM, Elmabrouk Abdalla FB, Elfageih MA, et al. Breast cancer patients in Libya: Comparison with European and central African patients. Oncol Lett 2011;2:323-30. [Crossref] [PubMed]
- Zarmouh A, Almalti A, Alzedam A, et al. Cancer incidence in the middle region of Libya: Data from the cancer epidemiology study in Misurata. Cancer Rep (Hoboken) 2022;5:e1448. [Crossref] [PubMed]
- Gusbi E, Elgriw N, Zalmat S, et al. Breast cancer in western part of Libya: Pattern and management (2003-2018). Libyan Journal of Medical Sciences 2020;4:65. [Crossref]
- Rakha EA, Reis-Filho JS, Baehner F, et al. Breast cancer prognostic classification in the molecular era: the role of histological grade. Breast Cancer Res 2010;12:207. [Crossref] [PubMed]
- Azim HA Jr, Partridge AH. Biology of breast cancer in young women. Breast Cancer Res 2014;16:427. [Crossref] [PubMed]
- Kim HJ, Kim S, Freedman RA, et al. The impact of young age at diagnosis (age< 40 years) on prognosis varies by breast cancer subtype: A US SEER database analysis. Breast 2022;61:77-83. [Crossref] [PubMed]
- Elidrissi Errahhali M, Elidrissi Errahhali M, Ouarzane M, et al. First report on molecular breast cancer subtypes and their clinico-pathological characteristics in Eastern Morocco: series of 2260 cases. BMC Womens Health 2017;17:3. [Crossref] [PubMed]
- Fourati A, Boussen H, El May MV, et al. Descriptive analysis of molecular subtypes in Tunisian breast cancer. Asia Pac J Clin Oncol 2014;10:e69-74. [Crossref] [PubMed]
- El-Hawary AK, Abbas AS, Elsayed AA, et al. Molecular subtypes of breast carcinoma in Egyptian women: clinicopathological features. Pathol Res Pract 2012;208:382-6. [Crossref] [PubMed]
- Belhadj A, Seddiki S, Belhadj A, et al. Prevalence and prognosis of molecular phenotypes in breast cancer patients by age: a population-based retrospective cohort study in western Algeria. Pan Afr Med J 2021;38:88. [Crossref] [PubMed]
- Bennis S, Abbass F, Akasbi Y, et al. Prevalence of molecular subtypes and prognosis of invasive breast cancer in north-east of Morocco: retrospective study. BMC Res Notes 2012;5:436. [Crossref] [PubMed]
- Cherbal F, Gaceb H, Mehemmai C, et al. Distribution of molecular breast cancer subtypes among Algerian women and correlation with clinical and tumor characteristics: a population-based study. Breast Dis 2015;35:95-102. [Crossref] [PubMed]
- Hashmi AA, Edhi MM, Naqvi H, et al. Clinicopathologic features of triple negative breast cancers: an experience from Pakistan. Diagn Pathol 2014;9:43. [Crossref] [PubMed]
- Chouchane L, Boussen H, Sastry KS. Breast cancer in Arab populations: molecular characteristics and disease management implications. Lancet Oncol 2013;14:e417-24. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Pankow JS, Vachon CM, Kuni CC, et al. Genetic analysis of mammographic breast density in adult women: evidence of a gene effect. J Natl Cancer Inst 1997;89:549-56. [Crossref] [PubMed]
- Gaceb H, Cherbal F, Bakour R, et al. Clinicopathological and Molecular Study of Triple-Negative Breast Cancer in Algerian Patients. Pathol Oncol Res 2018;24:297-308. [Crossref] [PubMed]
- Galukande M, Wabinga H, Mirembe F, et al. Molecular breast cancer subtypes prevalence in an indigenous Sub Saharan African population. Pan Afr Med J 2014;17:249. [Crossref] [PubMed]
- Deressa BT, Cihoric N, Badra EV, et al. Breast cancer care in northern Ethiopia - cross-sectional analysis. BMC Cancer 2019;19:393. [Crossref] [PubMed]
- Heer E, Harper A, Escandor N, et al. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob Health 2020;8:e1027-37. [Crossref] [PubMed]
- Ghiasvand R, Adami HO, Harirchi I, et al. Higher incidence of premenopausal breast cancer in less developed countries; myth or truth? BMC Cancer 2014;14:343. [Crossref] [PubMed]
- Bhikoo R, Srinivasa S, Yu TC, et al. Systematic review of breast cancer biology in developing countries (part 2): asian subcontinent and South East Asia. Cancers (Basel) 2011;3:2382-401. [Crossref] [PubMed]
- Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol 2019;30:1194-220. Erratum in: Ann Oncol 2019 Oct 1;30(10):1674. Ann Oncol. 2021 Feb;32(2):284. [Crossref] [PubMed]
- El-Habbash MM, Alwindi AA. Survival of breast cancer in very young women <35 years treated in Tripoli/Libya. Pan Arab Journal of Oncology 2013;28-31.
- Blamey RW, Hornmark-Stenstam B, Ball G, et al. ONCOPOOL - a European database for 16,944 cases of breast cancer. Eur J Cancer 2010;46:56-71. [Crossref] [PubMed]
- Kakarala M, Rozek L, Cote M, et al. Breast cancer histology and receptor status characterization in Asian Indian and Pakistani women in the U.S.--a SEER analysis. BMC Cancer 2010;10:191. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012;379:432-44. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011;378:771-84. [Crossref] [PubMed]
- Spitale A, Mazzola P, Soldini D, et al. Breast cancer classification according to immunohistochemical markers: clinicopathologic features and short-term survival analysis in a population-based study from the South of Switzerland. Ann Oncol 2009;20:628-35. [Crossref] [PubMed]
- Rezai M, Kellersmann S, Knispel S, et al. Translating the concept of intrinsic subtypes into an oncoplastic cohort of more than 1000 patients - predictors of recurrence and survival. Breast 2015;24:384-90. [Crossref] [PubMed]
- Yersal O, Barutca S. Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J Clin Oncol 2014;5:412-24. [Crossref] [PubMed]
- Gaibar M, Beltrán L, Romero-Lorca A, et al. Somatic Mutations in HER2 and Implications for Current Treatment Paradigms in HER2-Positive Breast Cancer. J Oncol 2020;2020:6375956. [Crossref] [PubMed]
- de Macêdo Andrade AC, Ferreira Júnior CA, Dantas Guimarães B, et al. Molecular breast cancer subtypes and therapies in a public hospital of northeastern Brazil. BMC Womens Health 2014;14:110. [Crossref] [PubMed]
- Majid RA, Hassan HA, Muhealdeen DN, et al. Breast cancer in Iraq is associated with a unimodally distributed predominance of luminal type B over luminal type A surrogates from young to old age. BMC Womens Health 2017;17:27. [Crossref] [PubMed]
- El Fatemi H, Chahbouni S, Jayi S, et al. Luminal B tumors are the most frequent molecular subtype in breast cancer of North African women: an immunohistochemical profile study from Morocco. Diagn Pathol 2012;7:170. [Crossref] [PubMed]
- Kondov B, Milenkovikj Z, Kondov G, et al. Presentation of the Molecular Subtypes of Breast Cancer Detected By Immunohistochemistry in Surgically Treated Patients. Open Access Maced J Med Sci 2018;6:961-7. [Crossref] [PubMed]
- Fallahpour S, Navaneelan T, De P, et al. Breast cancer survival by molecular subtype: a population-based analysis of cancer registry data. CMAJ Open 2017;5:E734-9. [Crossref] [PubMed]
- Nyagol J, Nyong'o A, Byakika B, et al. Routine assessment of hormonal receptor and her-2/neu status underscores the need for more therapeutic targets in Kenyan women with breast cancer. Anal Quant Cytol Histol 2006;28:97-103. [PubMed]
Cite this article as: Abousahmeen A, Saud MAB, Elrais S, Kashbour M, Abuhlaiga M, Al-Aqmar DM, Al-Shareef JM. Molecular subtypes and clinicopathological features of breast cancer in Libya: a first glance. Ther Radiol Oncol 2023;7:15.


