Childhood medulloblastoma in Morocco (middle-income country): therapeutic outcomes and survival
Highlight box
Key findings
• The study found that the overall survival rates for patients with medulloblastoma in Morocco were low, with a 5-year overall survival rate of 50%.
• The study also found that the delay radiotherapy and the presence of metastasis were significant prognostic factors.
What is known and what is new?
• There is limited information available on childhood medulloblastoma in Morocco.
• The study provides important information on the clinical profile and treatment outcomes of children with medulloblastoma in Morocco. But more research is needed to fully understand.
What is the implication, and what should change now?
• A better understanding of the therapeutic outcomes and survival rates for this disease in Morocco.
• It would be useful to improve the management of childhood medulloblastoma in Morocco and other low and middle-income countries.
Introduction
Medulloblastoma is one of the most common malignant brain tumors of childhood, comprising about 20% of all pediatric tumors (1-5). The incidence of medulloblastoma is estimated to be 0.7 per 100,000 children per year, with a male predominance (4). The improvements in the cure and the quality of survival for patients with cancer that have been made over the last three decades are impressive. However, the management of medulloblastoma in low and middle-income countries (LMIC) is difficult due to the expensive diagnostic technology such as computed tomography (CT) and/or magnetic resonance imaging (MRI), complicated neurosurgical techniques, and skilled neurosurgeons, as well as the expensive radiation therapy and lack of radiation centers and skilled radiotherapists. As a result, the therapy outcomes for medulloblastoma in LMIC are characterized by low survival rates and increased mortality. In Morocco, according to the Great Casablanca Register of Cancer, more than 538 cases of brain tumors have been reported in children under 15 years old, with rate of brain tumors accounting for 16% of cases (6). National epidemiological studies of primary brain tumors found that medulloblastoma and pilocytic astrocytoma were the most common types, accounting together for nearly half of the cases (7,8). This study aims to create a clinical profile and describe therapy outcomes and survival rates for patients with this disease. We present this article in accordance with the STROBE reporting checklist (available at https://tro.amegroups.com/article/view/10.21037/tro-22-39/rc).
Methods
Patients and clinical data
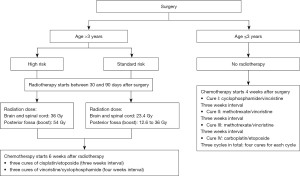
In a retrospective approach, we reviewed the medical records of 49 patients with medulloblastoma, diagnosed and treated at the University Hospital Ibn Sina of Rabat between January 2010 and December 2019. Only patients with histological confirmation according to the World Health Organization (WHO) classification 2007 and aged under 18 years at diagnosis were included (9). Clinical data and follow-up were collected from the medical records, including age, gender, the reason for consultation, symptoms, neuroimaging findings, the extent of resection, craniospinal fluid shunting, time from surgery to radiotherapy, duration of radiotherapy, duration of chemotherapy, and treatment results. the postoperative cerebrospinal fluid examination was not routinely performed. The risk stratification was categorized by age, tumor residual size, metastatic disease, and histologic subtype (Table 1). The treatment plan included a combination of surgery, radiotherapy, and chemotherapy (Figure 1).
Table 1
| High risk |
| Residual tumor >1.5 cm2 |
| Metastasis disease |
| Large cell or an anaplastic subtype |
| ≤3 years of age |
| Standard risk |
| Residual tumor ≤1.5 cm2 |
| Classic or desmoplastic subtype |
| >3 years of age |
Statistical analysis
The qualitative variables were expressed as percentages, while the quantitative variables were presented as means with standard deviations or medians with interquartile ranges. The patients were followed up with routine clinical and radiological examinations, and the data were fed into statistical software. Statistical analysis was carried out using SPSS. Overall survival rates were measured from the date of diagnosis to the date of death or last follow-up. Survival curves were constructed using the Kaplan-Meier method, and differences in overall survival between subgroups of patients were analyzed using the log-rank test. A P value (P≤0.05) was considered statistically significant.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee for biomedical research (CERB), Faculty of Medicine and Pharmacy Rabat Rabat, Mohamed V University/ethics board of AF 69/22, and informed consent was taken from the legal representatives of all the patients.
Results
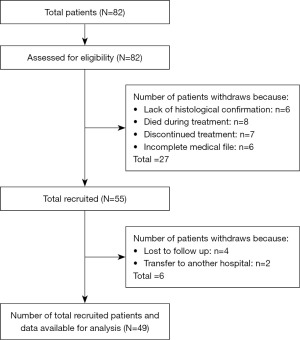
A total of 82 patients with medulloblastoma were referred to the Paediatric Oncology and Hematology Department of Ibn Sina University Hospital. Of these, 33 were excluded from the study for various reasons: 6 due to lack of histological confirmation, 8 died during treatment, 7 discontinued treatments, 4 were lost to follow-up, 6 had an incomplete medical file, and 2 were transferred to another hospital. The total number of patients recruited with available data was 49 patients (Figure 2).
Clinical and radiological characterization
The study included 49 children ranging in age from 2 to 16 years, with a median age of 8 years. Forty-three children were over 3 years old. The study included 27 males and 22 females, constituting a sex ratio (male/female) of 1.23, with a slight male preponderance (55.1% male versus 44.9% female). The most common reason for consultation was raised intracranial tension (headache, vomiting, and nausea), which was reported by 75.5% of patients. Cerebellar symptoms were the second most common, reported by 46.9% of patients. The primary symptoms were headache, nausea, and vomiting, which were reported by 37 patients. Secondly, cerebellar dysfunction symptoms were reported by 23 patients, and vision dysfunction symptoms (diplopia, strabismus, and decreased visual acuity) were reported by 10 patients. The mean time between the onset of symptoms and consultation was 60 days (range, 30–120 days). Among the medulloblastoma subtypes in our cohort, there were 24 cases of the classic variant, 11 cases of the nodular/desmoplastic variant, and 2 cases of the anaplastic/large cell variant. No cases of the extensive nodular variant were observed. Vermian localization was noted in 40 patients, while hemispheric localization was observed in 8 patients. At the time of diagnosis, there were 9 cases of metastasis, which increased to 37 patients in the post-therapy period. Sixteen patients had tumor residues greater than 1.5 cm². Other results are summarized in Table 2.
Table 2
| Characteristics | Values |
|---|---|
| Gender | |
| Male | 27 (55.1) |
| Female | 22 (44.9) |
| Sex-ratio (male/female) | 1.23 |
| Age (years) | 8.09 [2, 16] |
| Interval age | |
| ≤3 years | 6 (12.2) |
| >3 years | 43 (87.8) |
| Mean time from the first symptom and consultation (days) | 60 [30, 120] |
| Symptoms | |
| Nausea and vomiting | 37 (75.5) |
| Headache | 37 (75.5) |
| Cerebellar dysfunction | 23 (46.9) |
| Vision dysfunction (diplopia, strabismus decrease in visual acuity) | 10 (20.4) |
| Facial paralysis | 4 (8.2) |
| Hydrocephalus | |
| Yes | 30 (61.2) |
| No | 19 (38.8) |
| Tumor location | |
| Vermis | 40 (81.6) |
| Cerebellar hemisphere | 8 (16.3) |
| No data | 1 (2.0) |
| Tumor residue | |
| >1.5 cm² | 16 (32.7) |
| ≤1.5 cm² | 25 (51.0) |
| No data | 8 (16.3) |
| Metastasis at the moment of diagnostic | |
| Yes | 9 (18.4) |
| No | 40 (81.6) |
| Histological type | |
| Classic | 24 (49.0) |
| Nodular/desmoplastic | 11 (22.4) |
| Anaplastic/large cell | 2 (4.1) |
| Not classified | 12 (24.5) |
| Post-therapeutic metastasis | |
| Yes | 37 (75.5) |
| No | 12 (24.5) |
| Prognosis | |
| Average risk | 22 (44.9) |
| High risk | 27 (55.1) |
| Neuroimaging post-therapeutic | |
| Complete remission | 29 (59.2) |
| Tumoral residue | 11 (22.4) |
| Metastasis | 9 (18.4) |
Data are presented as n (%) or median [interquartile range].
Treatment and outcome characterization
A total of 37 patients underwent a preoperative ventriculoperitoneal shunt and 9 patients underwent a ventriculocisternostomy. All patients underwent surgery, with complete resection performed in 17 patients, subtotal resection in 21 patients, and biopsy in 11 patients. However, tumor resection was incomplete in 32 patients, 9 of whom had metastasis. A second surgery was necessary for 6 patients due to local relapse. The average time between surgery and radiotherapy was 92 days (ranging from 31–336 days). All patients over 3 years old received radiotherapy, which included spinal and brain treatment with doses ranging from 24 to 36 Gy, as well as posterior fossa irradiation with doses ranging from 18 to 54 Gy. The mean duration of radiotherapy was 45 days (ranging from 36–72 days). All patients received chemotherapy in the postoperative period, which included a combination of vincristine, cisplatin, etoposide, cyclophosphamide, and methotrexate. The average duration was 29 weeks (ranging from 14–39 weeks). Thirty-five patients developed neutropenia during chemotherapy. There were complete responses in 31 patients, a partial response in one patient, stable disease in 6 patients, and progression in 11 patients. Post-therapy neuroimaging was performed on all patients, including 16 cases of relapse and 9 cases of metastasis. The results are summarized in Table 3.
Table 3
| Characteristics | Values |
|---|---|
| Type of derivation | |
| Ventriculoperitoneal shunt | 37 (75.5) |
| Ventriculocisternostomy | 9 (18.4) |
| No derivation | 3 (6.1) |
| Surgery | |
| Yes | 49 (100.0) |
| No | 0 (0.0) |
| Tumoral resection | |
| Total | 17 (34.7) |
| Subtotal | 21 (42.9) |
| Biopsy | 11 (22.4) |
| The median duration between surgery and radiotherapy (days) | 133 [65, 161] |
| Radiotherapy | |
| Yes | 42 (85.7) |
| No | 7 (14.3) |
| Spine and brain doses | |
| 36 Gy | 33 (67.3) |
| 24 Gy | 2 (4.1) |
| No data | 14 (28.6) |
| Posterior fossa boost | |
| 18 Gy | 31 (63.3) |
| 54 Gy | 3 (6.1) |
| No data | 15 (30.6) |
| Mean duration of radiotherapy (days) | 45 [36, 72] |
| Chemotherapy | |
| Age ≤3 years | 6 (12.2) |
| Age >3 years | 43 (87.8) |
| Agents | |
| Etoposide | 46 (93.9) |
| Cisplatin | 38 (77.6) |
| Vincristine | 41 (83.7) |
| Methotrexate | 5 (10.2) |
| Endoxan | 41 (83.7) |
| Carboplatin | 14 (28.6) |
| The median duration of chemotherapy (weeks) | 20 [18, 32] |
| Response to chemotherapy | |
| Complete response | 31 (63.3) |
| Partial response | 1 (2.0) |
| Stable disease | 6 (12.2) |
| Progression | 11 (22.4) |
| Neutropenia | |
| Yes | 35 (71.4) |
| No | 13 (26.5) |
| No data | 1 (2.0) |
| The mean duration between surgery and chemotherapy (days) | 42.4 [12, 67] |
| Reoperated patients | |
| Yes | 5 (10.2) |
| No | 44 (89.8) |
| Recurrence rates | |
| Yes | 16 (32.7) |
| No | 33 (67.3) |
| Type of recurrence | |
| Local | 6 (12.2) |
| Distant | 10 (20.4) |
Data are presented as n (%) or median [interquartile range].
Survival analysis
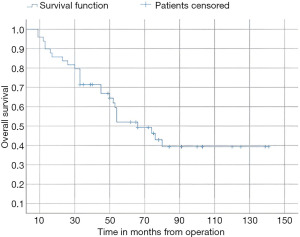
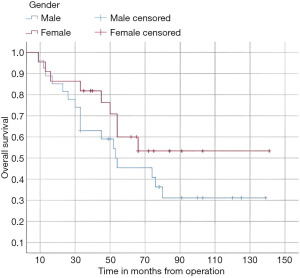
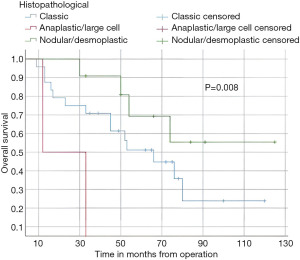
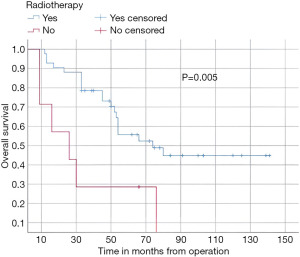
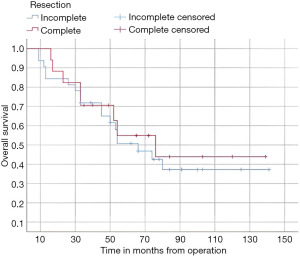
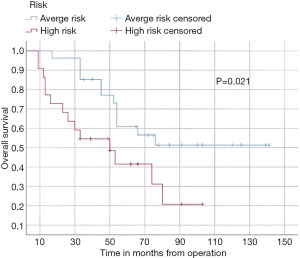
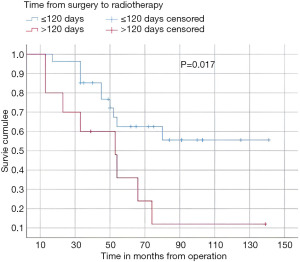
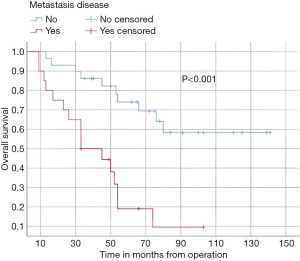
In this study, the median follow-up time was 43.5 months (range 22 to 70 months). The average overall survival of all patients after 5 years was 66 months with a 95% confidence interval (CI): 45.2–86.7. The overall survival rates after 1 year, 2 years, and 5 years were 94%, 84%, and 52%, respectively (Figure 3). We observed that the mean duration of overall survival was 72 months in males, while the mean duration in females was 94 months (P=0.19) (Figure 4). The 5-year overall survival based on histology was 51.3%, 0%, and 69.7% for classic, anaplastic/large cell, and nodular/desmoplastic types, respectively (P=0.008). The anaplastic type had the worst overall survival rates (Figure 5). We found that patients treated with radiotherapy had the best average overall survival (87.9 months) with a statistically significant P value (P=0.005), while others had an average overall survival of 34.5 months (Figure 6). The 5-year overall survival rates of complete and incomplete resection were approximately 55% and 51% respectively, with a mean survival of 76 months for complete resection versus 66 months for incomplete resection (P=0.67) (Figure 7). By type of risk, high risk patients had the worst 5-year overall survival rates (27.3%) versus 55.5% for standard risk (P=0.021) (Figure 8). The time from surgery to radiotherapy had a statistically significant P value if the duration was more than 120 days. We found that patients who started radiotherapy within 120 days had the best overall survival rates (average survival =99.4 months) compared to those who started later than 120 days (average survival =54.5 months) (P=0.017) (Figure 9). We also found that patients with metastasis had the worst average survival rates (43 months) compared to patients without metastasis (average survival rates =103.2 months) (P<0.001) (Figure 10). The main results are summarized in Table 4.
Table 4
| Characteristics | Overall survival after 1 year, n (%) | Overall survival after 5 years, n (%) | Mean/average [95% CI], months | Log-rank (Mantel_cox), P |
|---|---|---|---|---|
| Gender | 0.19 | |||
| Male (n=27) | 25 (92.6) | 10 (37.0) | 71.7 [52.37, 91] | |
| Female (n=22) | 21 (95.5) | 11 (50.0) | 93.9 [70.69, 117.1] | |
| Interval age | 0.5 | |||
| >3 years (n=43) | 40 (93.0) | 18 (41.9) | 82.7 [66.18, 99.23] | |
| ≤3 years (n=6) | 5 (83.3) | 3 (50.0) | 52 [29.18, 74.82] | |
| Radiotherapy | 0.005 | |||
| Yes (n=42) | 41 (97.6) | 19 (45.2) | 87.9 [71.5, 104.24] | |
| No (n=7) | 5 (71.4) | 2 (28.6) | 34.57 [12.4, 56.6] | |
| Metastasis disease | <0.001 | |||
| Yes (n=20) | 17 (85.0) | 3 (15.0) | 43.09 [30.4, 55,69] | |
| No (n=29) | 28 (96.6) | 17 (58.6) | 103.26 [84.7, 121.82] | |
| Resection | 0.67 | |||
| Complete (n=17) | 17 (100.0) | 7 (41.2) | 76 [40.2, 111.7] | |
| Incomplete (n=32) | 29 (90.6) | 14 (43.7) | 66 [41.2, 90.7] | |
| Risk stratification | 0.021 | |||
| Standard risk (n=27) | 26 (96.3) | 15 (55.5) | 95.8 [76.9, 114.6] | |
| High risk (n=22) | 19 (86.4) | 6 (27.3) | 52 [36.9, 67.7] | |
| Time from surgery to RT | 0.017 | |||
| ≤120 days (n=27) | 26 (96.3) | 13 (48.1) | 99.4 [79.5, 119.3] | |
| >120 days (n=10) | 8 (80.0) | 3 (30.0) | 54.5 [30.2, 78.7] |
CI, confidence interval; RT, radiotherapy.
Long-term complications
In our series of 24 surviving patients, 7 patients had no complications. The others suffered from endocrine, neurocognitive dysfunction, neurosensory, and neurological dysfunction. All results are summarized in Table 5.
Table 5
| Characteristics | Values, n (%) |
|---|---|
| No complications | 7 (29.2) |
| Neuro-sensory dysfunction (vertigo, cataract, strabismus, diplopia) | 6 (25.0) |
| Neurocognitive dysfunction (memory disorder, learning difficulty, concentration) | 4 (16.7) |
| Neurological dysfunction (ataxia, facial nerve paralysis) | 4 (16.7) |
| Endocrine (delay puberty, growth hormone insufficiency) | 3 (12.5) |
Discussion
Medulloblastoma is a common malignant brain tumor in children, with 80% of cases occurring in children (10). The aim of the study is to describe therapy outcomes and survival rates for patients with medulloblastoma. The median age at diagnosis is 5 to 6 years. The tumor location is principally vermian in childhood (11), and male preponderance was reported with a sex ratio of 1.7 (12). Our results are mostly similar to the results found previously. In our study, we found a small male preponderance, with 55.1% male and 44.9% female, and a sex ratio of 1.23. The median age at diagnosis was 8 years old, and 46 children were over 3 years old. The most common tumor location was the cerebellar vermis (81.6%), followed by the cerebellar hemisphere (16.3%). These results are consistent with previous Moroccan studies (7,8). Some authors consider age as a prognostic factor, with infants with medulloblastoma generally having poorer outcomes than older children and adults, due to the absence of postoperative radiotherapy and the delayed diagnosis (13,14). In our data, the poor prognosis was noted in patients younger than 3 years, with overall survival rates after 5 years less than 50%. Our data also revealed that the most common clinical manifestation was raised intracranial tension symptoms, such as headache, vomiting, and nausea, in 37 cases. These results are similar to those previously found (15). The role of sex in predicting outcomes of medulloblastoma patients is still controversial, with some studies showing a better prognosis for females (16,17), while others find no influence of gender (18). Our data showed that females had a slight increase in the mean overall survival after 5 years, although this was not statistically significant. The importance of tumor resection was recognized by Harvey Cushing, who noted an increased survival time in patients who underwent total resection compared to biopsy alone (19). On the other hand, gross total resection and near-total resection have not been proven to have similar results (20). In our data, there is no association between overall survival rates and the extent of resection. The interval between surgery and postoperative radiation should not exceed 90 days (16,21). Patients had poorer outcomes with treatment and intervals superior to 90 days (18,22). In a randomized trial, time to radiotherapy >49 days showed a trend toward poorer progression-free survival in univariate tests (23). For patients treated with pre-radiation chemotherapy, it is recommended that radiotherapy should commence within 110 days of surgery. The event-free survival was significantly worse for patients starting radiotherapy within 110 days compared to those who started radiotherapy later (P=0.004) (24). In our case, the time from surgery to radiotherapy had a statistically significant value for patients who had radiotherapy within 120 days compared to those who started radiotherapy later than 120 days (P=0.017) overall survival after 5 years was 62% versus 32%. Patients did not undergo timely radiotherapy because the fact that there is only one university radiotherapy center located in the Moroccan capital, which leads to a high flow of patients and postponement of the appointments, as well as several socio-economic issues such as financial and transport difficulties, treatment abandonment, and parents misunderstandings about the disease.
Postoperative radiotherapy was a favorable prognostic factor (P=0.005), and the 5-year overall survival rates were 55%. The seven patients who did not receive radiotherapy in our cohort due to various reasons (age under 3 years, dorsal position difficulties) had worse outcomes with overall survival rates after 5 years at 28%. These results are consistent with the reported data (18,25). Histologically, the classic type was the most prevalent (n=24), while the desmoplastic/nodular type had the best outcomes with 5 years overall survival rates of 69% (P=0.008). Our results matched those of Rutkowski et al. (26). The stage of metastasis influenced 5 years overall survival rates (15%) versus no metastasis (58.6%), P<0.001. The same results were noted in many series (24,27).
Conclusions
The reported results on survival rates were encouraging and close to those found in other studies, finally, this retrospective study provides epidemiological data and the main features of this pathology in Morocco, but we are unable to perform a molecular classification, which is important for a better understanding of the pathology and for providing the best therapeutic advances to patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tro.amegroups.com/article/view/10.21037/tro-22-39/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tro.amegroups.com/article/view/10.21037/tro-22-39/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee for biomedical research (CERB), Faculty of Medicine and Pharmacy Rabat Rabat, Mohamed V University/ethics board of AF 69/22, and informed consent was taken from the legal representatives of all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pui CH, Gajjar AJ, Kane JR, et al. Challenging issues in pediatric oncology. Nat Rev Clin Oncol 2011;8:540-9. [Crossref] [PubMed]
- Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol 2014;16:iv1-63. [Crossref] [PubMed]
- Smoll NR, Drummond KJ. The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J Clin Neurosci 2012;19:1541-4. [Crossref] [PubMed]
- McKean-Cowdin R, Razavi P, Barrington-Trimis J, et al. Trends in childhood brain tumor incidence, 1973-2009. J Neurooncol 2013;115:153-60. [Crossref] [PubMed]
- Farwell JR, Dohrmann GJ, Flannery JT. Medulloblastoma in childhood: an epidemiological study. J Neurosurg 1984;61:657-64. [Crossref] [PubMed]
- IRC. Registre des cancers du Grand Casablanca. Recherche en Cancerologie. Available online: https://www.irc.ma/registres-observatoires/registre-des-cancers/registre-populationnel/registre-des-cancers-du-grands-casablanca
- Harmouch A, Taleb M, Lasseini A, et al. Epidemiology of pediatric primary tumors of the nervous system: a retrospective study of 633 cases from a single Moroccan institution. Neurochirurgie 2012;58:14-8. [Crossref] [PubMed]
- Karkouri M, Zafad S, Khattab M, et al. Epidemiologic profile of pediatric brain tumors in Morocco. Childs Nerv Syst 2010;26:1021-7. [Crossref] [PubMed]
- Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:97-109. [Crossref] [PubMed]
- Yazigi-Rivard L, Masserot C, Lachenaud J, et al. Childhood medulloblastoma. Arch Pediatr 2008;15:1794-804. [Crossref] [PubMed]
- Padovani L, Sunyach MP, Perol D, et al. Common strategy for adult and pediatric medulloblastoma: a multicenter series of 253 adults. Int J Radiat Oncol Biol Phys 2007;68:433-40. [Crossref] [PubMed]
- Lannering B, Rutkowski S, Doz F, et al. Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard-risk medulloblastoma: results from the randomized multicenter HIT-SIOP PNET 4 trial. J Clin Oncol 2012;30:3187-93. [Crossref] [PubMed]
- Cornu P, Chatellier G, Fauchon F, et al. The prognosis of medulloblastoma in adults. Neurochirurgie 1990;36:218-24. [PubMed]
- Ismaili K, Sariban E, Otten J, et al. Medulloblastoma: analysis of 14 cases treated at the J. Bordet Institute and literature review. Rev Med Brux 1992;13:51-60. [PubMed]
- Wang C, Yuan XJ, Jiang MW, et al. Clinical characteristics and abandonment and outcome of treatment in 67 Chinese children with medulloblastoma. J Neurosurg Pediatr 2016;17:49-56. [Crossref] [PubMed]
- Weil MD, Lamborn K, Edwards MS, et al. Influence of a child's sex on medulloblastoma outcome. JAMA 1998;279:1474-6. [Crossref] [PubMed]
- Riffaud L, Saikali S, Leray E, et al. Survival and prognostic factors in a series of adults with medulloblastomas. J Neurosurg 2009;111:478-87. [Crossref] [PubMed]
- Paulino AC. Current multimodality management of medulloblastoma. Curr Probl Cancer 2002;26:317-56. [Crossref] [PubMed]
- Kunschner LJ. Harvey Cushing and medulloblastoma. Arch Neurol 2002;59:642-5. [Crossref] [PubMed]
- Zeltzer PM, Boyett JM, Finlay JL, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children's Cancer Group 921 randomized phase III study. J Clin Oncol 1999;17:832-45. [Crossref] [PubMed]
- Taillandier L, Blonski M, Carrie C, et al. Medulloblastomas Rev Neurol (Paris) 2011;167:431-48. review. [Crossref] [PubMed]
- Kann BH, Park HS, Lester-Coll NH, et al. Postoperative Radiotherapy Patterns of Care and Survival Implications for Medulloblastoma in Young Children. JAMA Oncol 2016;2:1574-81. [Crossref] [PubMed]
- Dietzsch S, Placzek F, Pietschmann K, et al. Evaluation of Prognostic Factors and Role of Participation in a Randomized Trial or a Prospective Registry in Pediatric and Adolescent Nonmetastatic Medulloblastoma - A Report From the HIT 2000 Trial. Adv Radiat Oncol 2020;5:1158-69. [Crossref] [PubMed]
- Taylor RE, Bailey CC, Robinson KJ, et al. Outcome for patients with metastatic (M2-3) medulloblastoma treated with SIOP/UKCCSG PNET-3 chemotherapy. Eur J Cancer 2005;41:727-34. [Crossref] [PubMed]
- Whelan HT, Krouwer HG, Schmidt MH, et al. Current therapy and new perspectives in the treatment of medulloblastoma. Pediatr Neurol 1998;18:103-15. [Crossref] [PubMed]
- Rutkowski S, Bode U, Deinlein F, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med 2005;352:978-86. [Crossref] [PubMed]
- Tait DM, Thornton-Jones H, Bloom HJ, et al. Adjuvant chemotherapy for medulloblastoma: the first multi-centre control trial of the International Society of Paediatric Oncology (SIOP I). Eur J Cancer 1990;26:464-9. [PubMed]
Cite this article as: Jaafari M, Razine R, Haroun AE, Tahiri Z, Hessissen L. Childhood medulloblastoma in Morocco (middle-income country): therapeutic outcomes and survival. Ther Radiol Oncol 2023;7:10.