
The impact of a mono-institutional experience in lung metastases treated with stereotactic body radiation therapy (SBRT): a retrospective analysis
Highlight box
Key findings
• Local control (LC), local progression-free survival, and overall survival evaluation in lung metastases stereotactic body radiation therapy (SBRT).
• Evaluation of toxicities and patient compliance to the treatment.
• Analysis of SBRT response of lung lesions from primary colorectal tumor cases.
What is known and what is new?
• SBRT for early-stage lung cancer has become one of the standard treatments but efficacy of SBRT for lung metastases have not been thoroughly evaluated, and its indication has not been defined as standard.
• SBRT in lung lesions showed efficacy in both responses and maintenance. Treatment with SBRT of lesions ≤5 cc at doses greater than 150 Gy biological equivalent dose_10Gy and with reduced volumes of therapy achieved better results in terms of LC and toxicity.
• Lower response rates for SBRT of lung metastases from primary colorectal tumor treated after 2 years from the end of chemotherapy.
What is the implication, and what would change now?
• SBRT of lung lesions may achieve a better result if performed earlier than in other therapeutic approaches.
IntroductionOther Section
Stereotactic body radiation therapy (SBRT) can deliver a very high dose to the tumor while minimizing the damage to the surrounding structures, in order to have a high local tumor control with acceptable normal tissue toxicity (1). Several studies (2,3) and European Society for Radiotherapy and Oncology (ESTRO) guidelines have reported on the efficacy of SBRT for early-stage lung cancer and this treatment has become one of the standard treatments for the disease (radiotherapy and surgery). Others (4-6) have reported on the use of SBRT for oligomets lung disease. Modern radiation technologies allow to perform SBRT as a high conformal radiation treatment planning that maximizes the dose within the target volume while optimizing the steep dose gradients beyond the target boundaries. This minimizes the dose to the surrounding organs at risk (OAR). The most important technical requirements for SBRT include modern linear accelerators with integrated image guidance solutions, sophisticated immobilization systems, advanced treatment planning software, and some level of adaptive patient realignment capabilities, such as robotic positioning technologies (7). Nevertheless, the efficacy of SBRT treating lung metastases have not been thoroughly evaluated, and its indication has not been defined as standard (5). SBRT for the treatment of metastases of solid tumors usually obtains local control (LC) rates up to 90–95%, depending on histology, results in colorectal metastases are less satisfactory (8).
It has been postulated that immunotherapy can be used with radiation to significantly improve outcomes in patients with advanced disease (abscopal effect). Additionally, it has been suggested that SBRT administered prior to immunotherapy can lead to the improved efficacy of the immunotherapy treatment (9). However, as immune checkpoint inhibitors (ICIs), they have been associated with a panel of specific inflammatory adverse events called immune-related adverse events, among which pneumonitis (10). Direct DNA damage and the production of reactive oxygen and nitrogen species leading to DNA damage and clonogenic death in alveolar epithelial cells are the main mechanisms of the radiation damage resulting in radiation pneumonitis (11). Anticancer molecules most commonly involved with radiation induce pulmonary fibrosis are taxanes, gemcitabine, and tyrosine kinase inhibitors. More recently, several cases of pulmonary fibrosis with ICIs have also been described (10). High dose SBRT, in some cases, can cause adverse effects as fatigue, dyspnea, rib fracture, esophagitis and pneumonitis (12). Compared to conventional radiotherapy, SBRT show a significantly lower risk of dyspnea, pneumonitis and esophagitis (13). This study investigates only pulmonary fibrosis as radiation induced toxicities, without looking for a potential correlation with immunotherapy.
The purpose of our study was to investigate LC, overall survival (OS) and local progression-free survival (LPFS) to determine the impact of SBRT lung treatment in metastatic lesions, focusing on histology, doses, volumes, techniques, and toxicities. A secondary end point estimated the impact of colorectal metastases stratified for the same parameters.
MethodsOther Section
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Since the study regards the statistical retrospective analysis of our clinical experience, it doesn’t require any ethical committee approval. Informed consent was taken from all the patients involved before any treatment.
Patient characteristics
Our retrospective analysis involved all patients age >18 years treated with SBRT for primary or metastatic lung tumors at UPMC Hillman Cancer Center San Pietro, located in Rome, between 2013 and 2020. The study population included 265 patients with, in total, 483 lesions (17% central, 83% peripheral) as described in Table 1. Patients’ gender was 55% female, 45% male; 34% of patients affected by non-small cell lung cancer (NSCLC) and 66% with metastases. Considering only the metastatic lung tumors, 37% of the patients presented localized colorectal metastases.
Table 1
| Characteristics | N [%] (n=265 patients, n=483 lesions) |
|---|---|
| Age (years), mean [range] | 72 [34–97] |
| Gender | |
| Male | 119 [45] |
| Female | 146 [55] |
| Histology | |
| Lung tumors | 163 [34] |
| Metastases | 320 [66] |
| Lung | 163 [34] |
| Gastrointestinal | 177 [37] |
| Gynecological | 29 [6] |
| Breast | 35 [7] |
| Prostate | 5 [1] |
| Mesenchymal | 34 [7] |
| Head & neck | 31 [7] |
| Bladder | 5 [1] |
| Location | |
| Central | 83 [17] |
| Peripheral | 400 [83] |
| Left upper lobe | 115 [24] |
| Left lower lobe | 91 [19] |
| Right upper lobe | 126 [26] |
| Right middle lobe | 48 [10] |
| Right lower lobe | 103 [21] |
According to the Radiation Therapy Oncology Group (RTOG) definition, our tumor classification defined central if the clinical target volume (CTV) was placed within 2 cm of the proximal bronchial tree or esophagus, and peripheral if the CTV did not meet the criteria for central.
Planning and treatment
Patients’ setup was supine, immobilized with BlueBAG Vacuum Cushions (Elekta), without abdominal compression and both arms above the head. Planning computed tomography (CT) scans were obtained with 1.25-mm slice thickness to acquire ten-phase 4-dimensional (4D) CT data sets.
Gross tumor volume (GTV) was defined based on the planning CT scan. The internal target volume (ITV) was generated from a review of all 10 phases from the 4D CT data.
Afterwards, a planning target volume (PTV) were created adding 3–5 mm margin to the ITV, depending of imaging protocol. A daily cone beam CT (CBCT) was obtained before each treatment to confirm tumor excursion and anatomy matching and 5 mm were added to avoid setup errors. When it was available the 4D CBCT, the margin from ITV to PTV was reduced to 3 mm. When the tumor displacement due to respiration was higher than 5 mm, we prefer a phase gating, which allows to deliver the radiation beam synchronously with the flow of the lung tumor. If tumor displacement was less than 5 mm, we enlarged our CTV to create a free breathing PTV.
Planning criteria and limiting doses to OAR for peripheral tumors were followed RTOG 0236 and RTOG 0618 studies. RTOG 0813 specifications for central tumors (14).
Treatment planning required the prescription isodose line to cover at least 98% of the PTV and at least 90% of the prescription dose covering 99% of the PTV.
All patients were treated using 6 MV-flattering filter free (FFF) photons on TrueBeamSTx linear accelerator (Varian medical System, Palo Alto, California) with volumetric modulated arc therapy (VMAT). Treatment planning systems (TPS) were Eclipse v.15 (Varian medical System, Palo Alto, California). Pulmonary heterogeneity had been taken into account by using the AAA algorithm for VMAT plans. Quality assurance (QA) was conducted for every plan before treatment using an ionization chamber and a 2-dimensional array.
Early data from Timmerman et al. (15) demonstrated increased toxicity, with the use of 60 to 66 Gy in 3 fractions. Our preferred fractionation schedule was in 3–5 fractions. Additionally, several alternative schedules were used early in our institutional SBRT experience. To allow for comparison among different doses, all radiation doses were expressed as biological equivalent dose (BED) according to the formula: BED = D × {1 + [d/(α/β)]}, assuming an α/β of 10 for lung lesions (BED_10Gy). The median BED with α/β =10 was 110 Gy BED_10Gy (range, 72–180 BED_10Gy).
All lesions treated were categorized into two different BED_10Gy (74% ≤150 Gy; 26% >150 Gy). Patients treated with BED_10Gy ≤150 Gy received the prescribed dose in 3, 4 or 5 fractions with total prescribed doses from 45 to 50 Gy. BED_10Gy >150 Gy were delivered using fractionation schemes based on 3 fractions with a total dose of 54 or 60 Gy. CTVs with volumes >5 cc were 30% instead of 70% of CTVs with volumes ≤5 cc (Table 2). All patients underwent motion management 4D CT during simulation; 34% of lesions were treated using a phase gating (mean 30–70% of breathing phases), adding an ITV to the GTV and the margin from phases, the other 66% were treated with an enlarged volume driven by 4D CT.
Table 2
| Characteristics | N [%] |
|---|---|
| Technique | |
| Phase gating | 165 [34] |
| Enlarged volume | 318 [66] |
| Neo/adjuvant therapies | |
| w Bevacizumab | 75 [16] |
| w/o Bevacizumab | 408 [84] |
| w Immunotherapy | 59 [12] |
| w/o Immunotherapy | 424 [88] |
| Volume | |
| Clinical target volume ≤5 cc | 338 [70] |
| Clinical target volume >5 cc | 145 [30] |
| Dose | |
| BED_10Gy ≤150 Gy | 357 [74] |
| BED_10Gy >150 Gy | 126 [26] |
w, with; w/o, without; BED, biological equivalent dose.
Follow-up and response assessment
Follow-up was generally undertaken at 4 weeks, and 2, 4, 6, 12 months following SBRT, and annually thereafter. A local recurrence was defined as a recurrence in proximity of the PTV. Disease progression was defined as a tumor recurrence in any part of the body. According to the Response Evaluation Criteria In Solid Tumor (RECIST, version 1.1), tumor recurrence was defined as a 20% increase in tumor size on the CT scan compared with the previous. In addition, a corresponding avid lesion on the positron emission tomography (PET) scan was required. Complete response was defined as the evanescence of the target lesion, and a partial response as a decrease in volume at least of 30% of the tumor.
Following the Common Terminology Criteria for Adverse Events (CTCAE, version 5), all cases of pulmonary fibrosis were scored as toxicities and were considered acute if occurring within 3 months from the first day of treatment and late if it occurred thereafter. Other toxicities as dyspnea, chest pain, laryngeal hemorrhage, peripheral sensory neuropathy, cough, productive cough, rib fracture, pleural effusion and gastro-esophageal reflux are investigated before treatment and during follow-ups.
Statistical analysis
LC was determined from the last day of SBRT to the date of local failure or the most recent follow-up. OS duration was defined as the last day of SBRT until the date of death or the last follow up. LPFS was measured from the last day of SBRT to local progression or death. The rates of LC, LPFS and OS were calculated using the Kaplan-Meier method. Prognostic factors such as metastatic disease, tumor size, dose fractionation, and tumor location (central vs. peripheral) were assessed to determine their impact on OS, LPFS, and LC.
The univariate analyses were followed by a multivariate analysis to determine LC and OS. The differences were evaluated using the log-rank test. SPSS software was used for statistical analysis (IBM, version 24.0) and statistical significance was defined as a P value of <0.05.
ResultsOther Section
LC, LPFS and OS
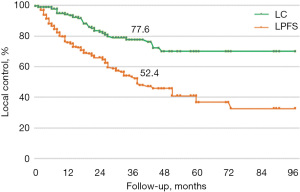
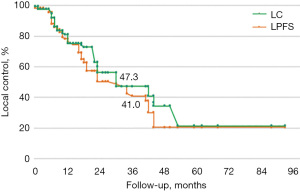
Median follow-up time was 56 months. LC, LPFS, and OS were calculated using the Kaplan-Meier method. In our sample, LC rates at 1, 3, and 5 years were 80%, 58%, and 44%, respectively (median LC was 44 months), LPFS was 65%, 41%, and 36% (median LPFS was 36 months), and OS was respectively 85%, 69%, and 56% (median OS was 64 months) (Figures 1-3).
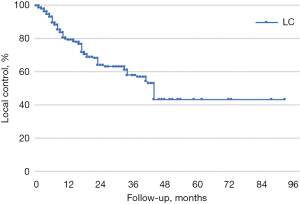
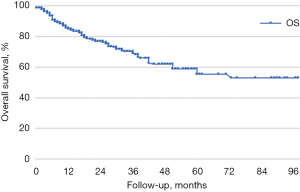
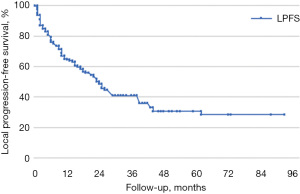
All lesions were stratified by BED_10Gy ≤150 Gy and BED_10Gy >150 Gy; CTV ≤5 cc and CTV >5 cc, treated using a phase gating technique or enlarged margins from GTV to ITV. Following the stratification there were no statistical differences in LC, OS, and LPFS rates (Table 3).
Table 3
| Category | Local control, n [%] | Chi-square | df | P value | |||
|---|---|---|---|---|---|---|---|
| 12 months | 24 months | 36 months | 48 months | ||||
| CTV | 0.890 | 1 | 0.765 | ||||
| ≤5 cc | 129 [81] | 43 [65] | 41 [58] | 20 [41] | |||
| >5 cc | 44 [78] | 19 [66] | 11 [57] | – | |||
| BED_10Gy | 0.560 | 1 | 0.454 | ||||
| ≤150 Gy | 126 [78] | 68 [66] | 38 [57] | 16 [40] | |||
| >150 Gy | 51 [88] | 25 [63] | 14 [59] | 10 [54] | |||
| ITV margins | 0.001 | 1 | 0.980 | ||||
| Enlarged | 113 [80] | 64 [64] | 39 [60] | 19 [47] | |||
| Phase gating | 53 [80] | 29 [67] | 15 [53] | 7 [37] | |||
CTV, clinical target volume; BED, biological equivalent dose; ITV, internal target volume.
There were no interruptions related to acute toxicity in the sample. Most common acute and late toxicities were pulmonary fibrosis (46%) followed by dyspnea (22%), cough (21%) and productive cough (11%). No Grade 3 or higher toxicities was founded. Acute and late Grade 2 pulmonary fibrosis were 2.5%.
Histology shows statistical differences in LC (P<0.01). Metastases arising from colorectal cancers are known to have a worse outcome compared to other primary subtypes. SBRT stratified by primary tumor, excluding lung lesions from primary colorectal tumor cases, shows increased LC rates at 1, 3, and 5 years (83%, 72% and 70%, respectively). Median LC was 56 months (P<0.01 by log-rank Mantel Cox test) (Figure 4).
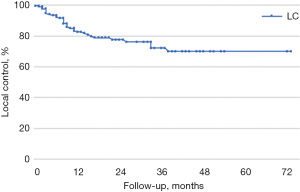
LC probability, excluding lung lesions from primary colorectal tumor, was also investigated as a function of prescribed dose, motion management, and CTV volume. LC in all lesions was statistically significant (P=0.020) if stratified by CTV ≤5 cc with a prescribed dose of BED_10Gy >150 Gy delivered with phase gating technique with a rate of 100% at 42 months.
LC probability in colorectal metastases was 74%, 41%, and 21%, at 1, 3, and 5 years after radiotherapy. If stratified by CTV ≤5 cc with a prescribed dose of BED_10Gy >150 Gy and delivered with phase gating technique, colorectal metastases indicate LC rate at 1, 3, and 5 years of 88%, 41%, and 27%, respectively.
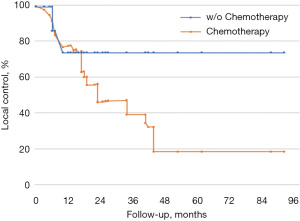
In order to investigate lung metastases from colorectal tumors we stratified our subgroup by chemotherapy cycles (Figure 5). Median chemotherapy duration before SBRT was 24 months (range, 6–72 months). The 2-year LC rate was 43% in lesions previously treated with several cycles of chemotherapy, and 75% in the subgroup treated with an early radiotherapy treatment with similar sample outcome with other histologies.
DiscussionOther Section
Lung metastases are frequent in 30–55% of cancer patients (16). Within the past year, a few trials have presented exciting data showing improved outcomes utilizing stereotactic radiosurgery (SRS) and SBRT in patients with various malignancies, defining an oligometastatic state as up to five metastases (17). Comparing the studies, our results mirror those mentioned above with excellent LC of over 80% at 1 year, with no relevant toxicities (13,15,17,18).
By examining the dose, volume, irradiation and histological techniques, the following was observed:
- Based on the various dose schemes employed we were able to show improved LC for doses with a BED_10Gy >150 Gy. Other BED_10Gy fractioning lesser than 100 Gy showed good but not statistically relevant outcomes.
- The study >5 cc volumes were not statistically relevant, but showed a worsening compared to ≤5 cc outcomes.
- No differences were found between phase gating and enlarged PTV since both irradiation techniques are based on the study of the movement in order to obtain a volume adequate to stereotaxic.
Results showed worse LC for colorectal metastases treated with a long period of chemotherapy, possibly due to greater radioresistance (19). Mean time of onset of colorectal metastases (n=117) was 2 years, 41% with mutated genes. Time from last chemotherapy and radiotherapy was around 9 months.
The increased radiation-resistance, likely due to clones selected during previous treatments, is confirmed once we assess the LC and LPFS curves gradients (Figures 6,7).
Limitation of the study
The limitations of our study due to the retrospective character, include selection bias. In addition, for what concern patients with metastatic disease, distant failure and death from non-pulmonary causes are significant competing factors and they must be considered.
ConclusionsOther Section
SBRT in lung lesions showed efficacy in both responses and maintenance. No significant toxicity was found, while good patient compliance was observed. Nonetheless, treatment with SBRT of lesions ≤5 cc at doses BED_10Gy greater than 150 Gy and with techniques that reduce the volume of therapy (using tolerance margins between 3–5 mm) achieved better results in terms of LC and toxicity.
This study shows a sub-population of patients who do not achieve the same results. In fact, in patients with primary tumor metastases from the gastrointestinal region, who have undergone previous pulmonary chemotherapy treatments (2 years), there are lower response rates than the rest of the examined sample.
The study suggests that SBRT treatment of lung lesions may achieve a better result if performed earlier than in other therapeutic approaches.
AcknowledgmentsOther Section
Funding: None.
FootnoteOther Section
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tro.amegroups.com/article/view/10.21037/tro-21-45/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Since the study regards the statistical retrospective analysis of our clinical experience, it doesn’t require any ethical committee approval. Informed consent was taken from all the patients involved before any treatment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Amsbaugh MJ, Woo SY. Stereotactic Radiation Therapy Techniques. In: Halperin EC, Wazer DE, Perez CA, et al. editors. Principles & Practice of Radiation Oncology. 7th edition. Alphen aan den Rijn, Wolters Kluwer; 2018:436-46.
- Zheng X, Schipper M, Kidwell K, et al. Survival outcome after stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: a meta-analysis. Int J Radiat Oncol Biol Phys 2014;90:603-11. [Crossref] [PubMed]
- Nagata Y, Kimura T. Stereotactic body radiotherapy (SBRT) for Stage I lung cancer. Jpn J Clin Oncol 2018;48:405-9. [Crossref] [PubMed]
- Alongi F, Arcangeli S, Filippi AR, et al. Review and uses of stereotactic body radiation therapy for oligometastases. Oncologist 2012;17:1100-7. [Crossref] [PubMed]
- Navarria P, De Rose F, Ascolese AM. SBRT for lung oligometastases: Who is the perfect candidate? Rep Pract Oncol Radiother 2015;20:446-53. [Crossref] [PubMed]
- Wild AT, Yamada Y. Treatment Options in Oligometastatic Disease: Stereotactic Body Radiation Therapy - Focus on Colorectal Cancer. Visc Med 2017;33:54-61. [Crossref] [PubMed]
- Sahgal A, Roberge D, Schellenberg D, et al. The Canadian Association of Radiation Oncology scope of practice guidelines for lung, liver and spine stereotactic body radiotherapy. Clin Oncol (R Coll Radiol) 2012;24:629-39. [Crossref] [PubMed]
- Nicosia L, Cuccia F, Alongi F. Reply to: The course of lung oligometastatic colorectal cancer may be a reflection of selection for treatment rather than an effect of stereotactic body radiotherapy. Strahlenther Onkol 2021;197:76-8. [Crossref] [PubMed]
- Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006;203:1259-71. [Crossref] [PubMed]
- Cousin F, Desir C, Ben Mustapha S, et al. Incidence, risk factors, and CT characteristics of radiation recall pneumonitis induced by immune checkpoint inhibitor in lung cancer. Radiother Oncol 2021;157:47-55. [Crossref] [PubMed]
- Türkkan G, Willems Y, Hendriks LEL, et al. Idiopathic pulmonary fibrosis: Current knowledge, future perspectives and its importance in radiation oncology. Radiother Oncol 2021;155:269-77. [Crossref] [PubMed]
- Thompson M, Rosenzweig KE. The evolving toxicity profile of SBRT for lung cancer. Transl Lung Cancer Res 2019;8:48-57. [Crossref] [PubMed]
- Li C, Wang L, Wu Q, et al. A meta-analysis comparing stereotactic body radiotherapy vs conventional radiotherapy in inoperable stage I non-small cell lung cancer. Medicine (Baltimore) 2020;99:e21715. [Crossref] [PubMed]
- Kong FM, Ritter T, Quint DJ, et al. Consideration of dose limits for organs at risk of thoracic radiotherapy: atlas for lung, proximal bronchial tree, esophagus, spinal cord, ribs, and brachial plexus. Int J Radiat Oncol Biol Phys 2011;81:1442-57. [Crossref] [PubMed]
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9. [Crossref] [PubMed]
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 2019;393:2051-8. [Crossref] [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007;2:S94-100. [Crossref] [PubMed]
- Jingu K, Matsushita H, Yamamoto T, et al. Stereotactic Radiotherapy for Pulmonary Oligometastases From Colorectal Cancer: A Systematic Review and Meta-Analysis. Technol Cancer Res Treat 2018;17:1533033818794936. [Crossref] [PubMed]
Cite this article as: Capone L, Antonia Allegretta S, Bianciardi F, Tolu B, Rea F, Giraffa M, Confaloni V, Raza GH, D’Ambrosio C, Cavallo F, Marchesano D, Grimaldi G, El Gahwary R, Cinelli E, Minniti G, Gentile P. The impact of a mono-institutional experience in lung metastases treated with stereotactic body radiation therapy (SBRT): a retrospective analysis. Ther Radiol Oncol 2023;7:13.



