Impact of adjuvant hysterectomy for bulky cervical cancer after definitive chemoradiotherapy with insufficient brachytherapy dose: a retrospective analysis
Introduction
Cervical cancer is the fourth most common gynecologic malignancy worldwide and the second most common cancer type in low- and middle-income countries. In 2018, 569,847 new cases of cervical cancer were reported and an estimated 311,365 deaths (1). In Taiwan, approximately 1,400 new cervical cancer cases were recorded and resulting in 650 deaths. The incidence rate ranks 9th among women; the mortality rate ranks 7th among women, according to the Taiwan Cancer Registry Annual Report, 2018 (2).
Locally advanced cervical cancer (LACC) is stage IB3, IIA2, IIB, IIIA, IIIB, IIIC and IVA according to International Federation of Gynecology and Obstetrics classification (FIGO 2018) (3). The recommended treatment is External beam radiation therapy (EBRT) with concurrent platinum-based chemotherapy, followed by intracervical brachytherapy (4). The latest National Comprehensive Cancer Network cervical guidelines (version 1.2021) suggested radiotherapy dose >85 Gy to point A. If high dose rate (HDR) brachytherapy is used, NCCN recommends giving five fractions of 6 Gy each time.
Due to a high recurrence rate after CCRT, up to 40.2%, AH is performed in some hospital (5). Although Gynecologic Oncology Group-71 trial seems to establish that AH is not required for FIGO stage IB cervical cancer after definitive radiotherapy (RT) (6), recent research seems to be challenging the conclusion. A retrospect data had evaluated the role of AH in patients with residual lesion who were treated with definitive CCRT. There was overall survival (OS) benefit of AH, although the local recurrence rate showed no different (7). A meta-analysis indicated that LACC patients who received CCRT with hysterectomy had significantly better OS (HR, 0.72; 95% CI: 0.56 to 0.91; P=0.007) and disease free survival (DFS) (HR, 0.72; 95% CI: 0.56 to 0.93; P=0.01) than those treated with CCRT alone (8).
Given the different anatomical structures of the cervix, the cumulative dose limitation of the rectum and bladder makes it difficult to irradiate sufficient doses to some bulky tumors. According to NCCN guidelines, AH was an option in the scenario of considering the initial extent of disease, poor response to CCRT or uterine anatomy precludes adequate coverage by brachytherapy. Therefore, this study aimed at the role of AH in patients who underwent brachytherapy with an insufficient dose. We present the following article in accordance with the STROBE reporting checklist (available at https://tro.amegroups.com/article/view/10.21037/tro-22-12/rc).
Methods
Patients
In this study, all patients with bulky cervical cancer received CCRT with insufficient brachytherapy dose between 2010 and 2020 were evaluated. Clinical information, date of diagnosis, age, tumor pathology, International Federation of Gynecology and Obstetrics classification (FIGO 2018) stage, tumor size, performance status (Eastern Cooperative Oncology Group, ECOG) were recorded. The primary outcome was disease specific survival (DSS). DSS is defined as the time from when the patient was diagnosed to death from cervical cancer. The secondary outcome was local control (LC). LC is defined as the time from diagnosis to cancer recurrence at primary site. Patients were censored at the last day of follow-up or death. Patients were excluded if they had received any neoadjuvant treatment or if they did not receive full treatment course in Chi Mei Medical Center, Liouying. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Institutional Review Board of Chi Mei Medical Center (IRB number: 11004-L02). Given the retrospective design of this study, informed consent was waived.
Treatment
All patient received CCRT, the decision for CCRT was reached through multidisciplinary team discussion. EBRT to entire uterus, cervix, upper vagina and pelvic lymph nodes regions with concurrent platin based chemotherapy. The EBRT dose was 45 Gy at least was delivered at 1.8–2 Gy per day, 5 days a week and the RT technique included three-dimensional conformal radiation therapy, volumetric modulated arc therapy, and helical tomotherapy. After EBRT, patients received HDR intracavitary brachytherapy (ICBT) with insufficient dose. The brachytherapy technique was two-dimensional (2D) classic brachytherapy. Included patients all received insufficient doses of brachytherapy, which was defined as brachytherapy doses less than 30 Gy. Cisplatin was administered on days 1, 22, 43 in the triweekly group. The other regimen was to prescribe cisplatin weekly to reduce acute toxicity. Performed adjuvant hysterectomy (AH) or not was based on the discussion in tumor board. Patient who had an extended disease, poor response to CCRT or uterine anatomy precludes adequate coverage by brachytherapy would be evaluated. Modified radical hysterectomy (class II hysterectomy) with pelvic lymph node was standard procedure. Biopsies and imaging studies not required between CCRT and surgery when brachytherapy doses are insufficient. Post-operative complication assessment is based on the need for long-term urological follow-up.
Statistical analysis
Pearson’s chi-square test for categorical variables and the Wilcoxon ranked sum test for continuous variables were performed for the distribution difference between cervical cancer patients receiving AH or not. The Kaplan-Meier method was applied to estimate 5-year DSS and LC rates. Log-rank tests were also used to compare the difference of survival curves. Cox proportional hazard models were fitted to estimate the effect of AH on survival, after adjusting to other confounding variables. All statistical analyses were performed using SAS 9.4 for Windows (SAS Institute, Inc., Cary, NC, USA). The P value less than 0.05 was considered statistically significant.
Results
Group characteristics
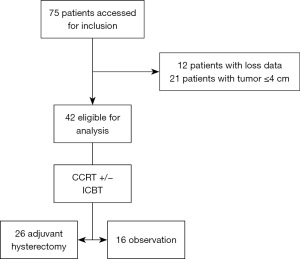
Forty-two patients were eligible for this study, 26 underwent AH and 16 received no adjuvant treatment (Figure 1). The median follow-up of the study was 39 months (range, 8–115 moths). The median total external beam radiation dose was 50.4 Gy (range, 45–60.4 Gy) and median HDR brachytherapy dose was 22.5 Gy (range, 0–25 Gy). Total cumulative dose (EQD2 with alpha-beta ratio =10) for point A was 72.5 Gy (range, 70–82.7 Gy).
The clinical and pathological characteristics are presented in Table 1. The median age was 64.5 years (range, 51–81 years) and 54 years (range, 45–60 years) in groups 1 and 2, respectively. Patients in the surgical group had lower FIGO stages but without statistically significant. Pathology, tumor size and ECOG were equally distributed between the two groups. The median radiation dose of (51.3 Gy) delivered during EBRT and dose of ICBT were similar in the two groups (Table 1). Reviewing the reasons for insufficient radiation dose, 7 patients did not receive brachytherapy because the brachytherapy devise could not be placed, and 35 patients had a reduced brachytherapy dose because the cumulative dose to the rectum and bladder was too high.
Table 1
| Variables | Adjuvant hysterectomy | P value* | |
|---|---|---|---|
| No (n=16) | Yes (n=26) | ||
| Age (years), median [IQR] | 64.5 [51–81] | 54.0 [45–60] | 0.070 |
| Age groups, n (%) | 0.032 | ||
| <65 | 8 (50.0) | 22 (84.6) | |
| ≥65 | 8 (50.0) | 4 (15.4) | |
| Stage (FIGO 2018), n (%) | 0.096 | ||
| I–II | 5 (31.3) | 15 (57.7) | |
| III–IV | 11 (68.7) | 11 (42.3) | |
| External beam radiotherapy dose (Gy), median (IQR) | 51.3 (46.8–60.4) | 51.3 (45–55.8) | 0.656 |
| Brachytherapy dose (Gy), median (IQR) | 22.5 (0–25) | 19.25 (0–24.5) | 0.173 |
| Total RT dose (EQD2) (Gy), median (IQR) | 76.4 (74.5–79.7) | 72.6 (69.0–80.0) | 0.158 |
| Total RT dose (EQD2) (Gy), n (%) | 0.630 | ||
| >80 | 4 (25.0) | 6 (23.1) | |
| 70–80 | 10 (62.5) | 13 (50.0) | |
| <70 | 2 (12.5) | 7 (26.9) | |
| Pathology, n (%) | >0.999 | ||
| Squamous cell carcinoma | 14 (87.5) | 22 (84.6) | |
| Adenocarcinoma | 2 (12.5) | 4 (15.4) | |
| Tumor size (cm), median (IQR) | 5.5 (4.7–6.1) | 4.9 (4.5–6.2) | 0.542 |
| ECOG, n (%) | 0.547 | ||
| 0–2 | 14 (87.5) | 25 (96.1) | |
| 3–4 | 2 (12.5) | 1 (3.9) | |
*, P values were estimated using the Chi-square test, Fisher exact test or Student t-test or Wilcoxon Mann-Whitney test. IQR, interquartile range; FIGO, International Federation of Gynecology and Obstetrics; RT, radiotherapy; ECOG, Eastern Cooperative Oncology Group.
Twenty-six patients received AH; 2 patients underwent preoperative biopsy, 3 patients received radical hysterectomy (Piver class III), 18 patients received modified radical hysterectomy (Piver class II), 5 patients received total hysterectomy (Piver class I). Primary tumor pathological complete response was observed in 13 (50%) in 26 patients.
Because surgery is an adjunct role, preoperative image studies are not necessary. Reviewing the cohort receiving AH, only 9 of 26 received imaging studies before surgery, and 3 of them achieved a clinical complete response (cCR) on imaging.
Outcome
Disease-specific survival (DSS) for the 42 patients in two groups is shown in Figure 2. There is a significant difference in disease survival rate. The 3-year DSS rate were 80% and 47% for AH and observation group respectively. The 5-year DSS rate were 75% and 36% for AH and observation group respectively. LC for the 42 patients in two groups is shown in Figure 3. There is no significant difference in LC rate.
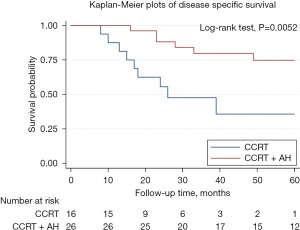
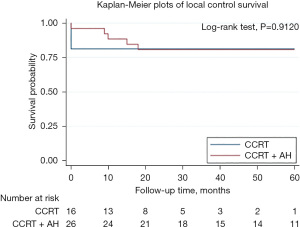
In univariate analysis, significant difference was found in prognostic factors related to DSS including AH (OR: 0.25, 95% CI: 0.09–0.71, P=0.001) and advance stage (OR: 3.17, 95% CI: 1.01–9.99, P=0.049). We then performed multivariate Cox regression analysis and we observed superior survival benefit of AH (OR: 0.27, 95% CI: 0.08–0.90, P=0.032) was reported as independent risk factors of DSS for bulky cervical cancer patients received CCRT (Table 2).
Table 2
| Variables | No. of events (n=15) | Crude | Adjusted | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI)a | P | |||
| Treatment | ||||||
| CCRT | 9 | Ref | Ref | |||
| CCRT + AH | 6 | 0.25 (0.09–0.71) | 0.001 | 0.27 (0.08–0.90) | 0.032 | |
| Total RT dose (EQD2) (Gy) | ||||||
| >80 | 6 | 3.74 (0.75–18.72) | 0.109 | 3.96 (0.69–22.80) | 0.124 | |
| 70–80 | 7 | 1.58 (0.33–7.64) | 0.568 | 1.28 (0.23–6.99) | 0.779 | |
| <70 | 2 | Ref | Ref | |||
| Age groups | ||||||
| <65 | 9 | Ref | Ref | |||
| ≥65 | 6 | 2.33 (0.82–6.58) | 0.112 | 1.17 (0.33–4.10) | 0.805 | |
| Tumor size | 15 | 1.09 (0.80–1.48) | 0.577 | 1.18 (0.73–1.90) | 0.495 | |
| Stage (FIGO 2018) | ||||||
| I–II | 4 | Ref | Ref | |||
| III–IV | 11 | 3.17 (1.01–9.99) | 0.049 | 1.63 (0.41–6.48) | 0.490 | |
| Pathology | ||||||
| SqCC | 14 | Ref | Ref | |||
| Adenocarcinoma | 1 | 0.37 (0.05–2.84) | 0.342 | 0.34 (0.03–4.06) | 0.395 | |
| ECOG | ||||||
| 0–2 | 13 | Ref | Ref | |||
| 3–4 | 2 | 3.60 (0.79–16.29) | 0.097 | 1.44 (0.26–8.06) | 0.677 | |
a, adjusted HR with all listed variables. DSS, disease specific survival; CCRT, concurrent chemoradiotherapy; AH, adjuvant hysterectomy; RT, radiotherapy; FIGO, International Federation of Gynecology and Obstetrics; SqCC, squamous cell carcinoma; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; Ref, reference.
Two urinary tract complications in radical hysterectomy (66.6%), 2 in modified radical hysterectomy (11.1%), 1 in total hysterectomy (20%) (Table 3).
Table 3
| Operation method | Patient number | Urinary complication | Complication rate (%) |
|---|---|---|---|
| Radical hysterectomy | 3 | 2 | 66.6 |
| Modified radical hysterectomy | 18 | 2 | 11.1 |
| Total hysterectomy | 5 | 1 | 20 |
Discussion
In this study, we found that compared with CCRT alone, patients with bulky cervical cancer who received AH after underdose brachytherapy had a significant DSS advantage in univariate Kaplan-Meier analysis (OR: 0.25, 95% CI: 0.09–0.71, P=0.001) and multivariate Cox regression analysis (OR: 0.27, 95% CI: 0.08–0.90, P=0.032), revealing the role of AH in patients with bulky cervical cancer who received underdose brachytherapy.
The treatment of bulky cervical cancer is still in progress. The GOG had completed a prospective randomized trial of irradiation with or without AH in patients with stage IB tumors larger than 4 cm. The 5-year local recurrence rate was lower in the AH arm (14% vs. 27%) but no OS benefit (6), but it was in the era of definitive RT. In the era of CCRT, there have been several studies of AH in LACC patients (9-14), they proclaimed the safety of AH after CCRT but lack of evidence of survival benefit of AH. Shim et al. used the National Cancer Database to determine the treatment pattern and survival impact of AH in patients with stage IB2 to IIA2 cervical cancer who received CCRT (15). For patients without nodal metastases, there was trend to improve in 4-year OS rates with the AH group comparing CCRT alone group (84.9% vs. 77.8%, P=0.072). In recent meta-analysis, Lu et al. reported that LACC patients who received CCRT with hysterectomy had significantly better OS (HR, 0.72; 95% CI: 0.56–0.91; P=0.007) and DFS (HR, 0.72; 95% CI: 0.56–0.93; P=0.01) than those treated with CCRT alone (8).
Although CCRT can significantly reduce the burden of tumors, and it may leave some residual CCRT-resistant cancer cells, which may become the source of recurrence or metastasis in the future. On the other hand, main tumor of cervical cancer relies on brachytherapy to give a sufficient dose. In the two-dimension brachytherapy era, prescribing to point A implying that only a four centimeters of width region has received enough therapeutic doses. Moreover, Walji et al. reported ICBT insertion was unsuccessful in 19 of 208 (9%) patients (16). Because of the above reasons, studies have reported the rate of residual disease on surgical specimen after CCRT ranges from 32% to 59% (6,17-21). In our investigation, primary tumor pathological complete response was observed in 13 (50%) in 26 patients, pointed out that about half of the patients had residual tumors after definitive CCRT. The age and FIGO stage were not equal in the two groups due to retrospective study. FIGO stage also had a significant effect on survival in univariate analysis, but only AH had a significant effect on survival in multivariate analysis. This result tells us that AH seems to be the most important influencing factor in terms of survival rate. Although AH did not increase LC, it is possible that AH reduced undetected local recurrence in the case of infrequent imaging examinations (22).
Radical hysterectomy resulting in a high rate of severe treatment-related morbidity and decreased quality of life, such as complications of urination and sexual/vaginal functioning. Modified radical hysterectomy, compare with radical hysterectomy, can reduce the inability of urination and sexual/vaginal functioning (23). In this study, when patient with bulky cervical tumor, only a small percentage (3/26, 11.5%) of patients undergo radical hysterectomy, and most patients received modified radical hysterectomy can still maintain relatively complete life functions.
Limitations of this study include its retrospective design with small sample-size. Second, a few patients were lost to follow-up prior to the completion of the cancer surveillance period. Third, this study points out that AH may improve the DSS of bulky cervical cancer but not LC, unable to explain where the advantages of DSS come from. This is the same in the previous study on whether the groups with residual tumors after cervical cancer CCRT received AH also showed OS’s benefit but no LC’s improvement (7). Finally, we also have to admit that younger age and better health may increase the chances of surgeon intervention. Although it did not reach statistical significance, it may be due to insufficient sample size.
In conclusion, AH after CCRT at insufficient doses may be a remedy although there was no significant increase in LC but DSS, modified radical hysterectomy (Piver class II) was suggested for lesser complication rate. Especially the younger patients with lower FIGO stage in this study. However, to do AH or not must be evaluated carefully based on the benefit of survival and the long-term complications. Further randomized control trial is needed to evaluate the survival benefit.
Acknowledgments
We thank Convergence CT offering help in reviewing and revising the manuscript for grammar and syntax.
Funding: This article was supported by the Department of Clinical Research of Chi Mei Hospital, Liouying (No. CLFHR11037).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tro.amegroups.com/article/view/10.21037/tro-22-12/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tro.amegroups.com/article/view/10.21037/tro-22-12/coif). All authors report that this study uses only an in-hospital research project, which aims to improve the research ethos of the hospital, and the research project can cover the costs of statistical experts for processing statistics, English editing costs, and paper publication costs. The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Taiwan Cancer Registry Annual Report. 2018. Available online: https://www.hpa.gov.tw/Pages/ashx/File.ashx?FilePath=~/File/Attach/13498/File_18062.pdf
- Bhatla N, Berek JS, Cuello Fredes M, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet 2019;145:129-35. [Crossref] [PubMed]
- Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol 2004;22:872-80. [Crossref] [PubMed]
- van Kol KGG, Ebisch RMF, Piek JMJ, et al. Salvage surgery for patients with residual disease after chemoradiation therapy for locally advanced cervical cancer: A systematic review on indication, complications, and survival. Acta Obstet Gynecol Scand 2021;100:1176-85. [Crossref] [PubMed]
- Keys HM, Bundy BN, Stehman FB, et al. Radiation therapy with and without extrafascial hysterectomy for bulky stage IB cervical carcinoma: a randomized trial of the Gynecologic Oncology Group. Gynecol Oncol 2003;89:343-53. [Crossref] [PubMed]
- Hass P, Eggemann H, Costa SD, et al. Adjuvant hysterectomy after radiochemotherapy for locally advanced cervical cancer. Strahlenther Onkol 2017;193:1048-55. [Crossref] [PubMed]
- Lu W, Lu C, Yu Z, et al. Chemoradiotherapy alone vs. chemoradiotherapy and hysterectomy for locally advanced cervical cancer: A systematic review and updated meta-analysis. Oncol Lett 2021;21:160. [Crossref] [PubMed]
- Fagotti A, Conte C, Stollagli F, et al. Radical Surgery in Advanced Cervical Cancer Patients Receiving Bevacizumab-Containing Chemotherapy: A "Real Life Experience". Int J Gynecol Cancer 2018;28:1569-75. [Crossref] [PubMed]
- Ferrandina G, Legge F, Fagotti A, et al. Preoperative concomitant chemoradiotherapy in locally advanced cervical cancer: safety, outcome, and prognostic measures. Gynecol Oncol 2007;107:S127-32. [Crossref] [PubMed]
- Ferrandina G, Ercoli A, Fagotti A, et al. Completion surgery after concomitant chemoradiation in locally advanced cervical cancer: a comprehensive analysis of pattern of postoperative complications. Ann Surg Oncol 2014;21:1692-9. [Crossref] [PubMed]
- Ferrandina G, Gambacorta A, Gallotta V, et al. Chemoradiation with concomitant boosts followed by radical surgery in locally advanced cervical cancer: long-term results of the ROMA-2 prospective phase 2 study. Int J Radiat Oncol Biol Phys 2014;90:778-85. [Crossref] [PubMed]
- Lu H, Wu Y, Liu X, et al. A prospective study on neoadjuvant chemoradiotherapy plus anti-EGFR monoclonal antibody followed by surgery for locally advanced cervical cancer. Onco Targets Ther 2018;11:3785-92. [Crossref] [PubMed]
- Tummers P, Makar A, Vandecasteele K, et al. Completion surgery after intensity-modulated arc therapy in the treatment of locally advanced cervical cancer: feasibility, surgical outcome, and oncologic results. Int J Gynecol Cancer 2013;23:877-83. [Crossref] [PubMed]
- Shim SH. Adjuvant hysterectomy in patients with locally advanced cervical cancer treated with concurrent chemoradiotherapy. J Gynecol Oncol 2019;30:e69. [Crossref] [PubMed]
- Walji N, Chue AL, Yap C, et al. Is there a role for adjuvant hysterectomy after suboptimal concurrent chemoradiation in cervical carcinoma? Clin Oncol (R Coll Radiol) 2010;22:140-6. [Crossref] [PubMed]
- Vandecasteele K, Makar A, Van den Broecke R, et al. Intensity-modulated arc therapy with cisplatin as neo-adjuvant treatment for primary irresectable cervical cancer. Toxicity, tumour response and outcome. Strahlenther Onkol 2012;188:576-81. [Crossref] [PubMed]
- Classe JM, Rauch P, Rodier JF, et al. Surgery after concurrent chemoradiotherapy and brachytherapy for the treatment of advanced cervical cancer: morbidity and outcome: results of a multicenter study of the GCCLCC (Groupe des Chirurgiens de Centre de Lutte Contre le Cancer). Gynecol Oncol 2006;102:523-9. [Crossref] [PubMed]
- Morice P, Rouanet P, Rey A, et al. Results of the GYNECO 02 study, an FNCLCC phase III trial comparing hysterectomy with no hysterectomy in patients with a (clinical and radiological) complete response after chemoradiation therapy for stage IB2 or II cervical cancer. Oncologist 2012;17:64-71. [Crossref] [PubMed]
- Jurado M, Martínez-Monge R, García-Foncillas J, et al. Pilot study of concurrent cisplatin, 5-fluorouracil, and external beam radiotherapy prior to radical surgery +/- intraoperative electron beam radiotherapy in locally advanced cervical cancer. Gynecol Oncol 1999;74:30-7. [Crossref] [PubMed]
- Houvenaeghel G, Lelievre L, Gonzague-Casabianca L, et al. Long-term survival after concomitant chemoradiotherapy prior to surgery in advanced cervical carcinoma. Gynecol Oncol 2006;100:338-43. [Crossref] [PubMed]
- Gui B, Valentini AL, Miccò M, et al. Cervical cancer response to neoadjuvant chemoradiotherapy: MRI assessment compared with surgery. Acta Radiol 2016;57:1123-31. [Crossref] [PubMed]
- Sun H, Cao D, Shen K, et al. Piver Type II vs. Type III Hysterectomy in the Treatment of Early-Stage Cervical Cancer: Midterm Follow-up Results of a Randomized Controlled Trial. Front Oncol 2018;8:568. [Crossref] [PubMed]
Cite this article as: Yen CH, Yang CC, Ho SY, Lee SW, Chen CC, Shieh LT. Impact of adjuvant hysterectomy for bulky cervical cancer after definitive chemoradiotherapy with insufficient brachytherapy dose: a retrospective analysis. Ther Radiol Oncol 2023;7:3.