
A narrative review of intensity-modulated proton therapy for head and neck cancer
Introduction
Head and neck cancer management involves a multi-disciplinary approach with radiation therapy as an essential treatment modality. Patients’ treatment options include single-modality surgery or radiation therapy, or surgery with adjuvant radiation with or without chemotherapy (1). The utilization of radiation therapy aims to improve overall survival (OS) and locoregional control (LRC); however, it is essential to limit adverse effects and improve the quality of life (QoL) of patients. More than two decades ago, implementing intensity-modulated radiation therapy (IMRT) enhanced radiation precision to targets while limiting the radiation dose to nearby structures, significantly reducing toxicities. Despite improvements, the inherent physical properties of photons still expose healthy tissue, leading to chronic toxicities that affect QoL (2). A novel radiation therapy was necessary for dose escalation while minimizing radiation to non-targeted tissue.
Physicist Robert Wilson originally proposed using proton therapy to target small volumes in 1946, and approximately a decade later, patients began treatment with proton therapy (3-5). The emergence of this particle therapy has theoretical benefits over photon therapy. In comparison to photons, proton therapy has little to no exit dose. Most of the heavy, charged proton particles deposit the radiation dose at a narrow range of tissue depth, called the “Bragg peak”. Localizing the Bragg peak to the selected tumor volume with minimal to no exit dose beyond the specified depth would significantly reduce toxicities and improve patients’ QoL. Intensity-modulated proton therapy (IMPT) is a mode of proton delivery that utilizes a pencil beam manipulated by electromagnetic radiation (6). While IMPT has expanded the therapeutic window and offers promising results, its utilization has been previously restricted by its costly expenses and limited availability. However, IMPT has become more prevalent in treating head and neck cancer as its accessibility and affordability have improved over the past decade.
In this review of IMPT in head and neck cancer, we aim to summarize recent developments in treatment planning and delivery and present clinical outcomes and toxicities based on head and neck cancer subsites. Limitations and future directions of IMPT in head and neck cancer will also be assessed. We present the following article in accordance with the Narrative Review reporting checklist (available at https://tro.amegroups.com/article/view/10.21037/tro-22-8/rc).
Methods
This review is not an attempt to complete a systematic review of IMPT; instead, we aim to summarize the literature on IMPT in head and neck cancer. To adequately review the literature, we conducted a PubMed search using the following search syntax: ((“intensity-modulated proton therapy” OR “IMPT” OR “pencil beam scanning” OR “scanning beam proton therapy”) AND “head and neck cancer”). Additional search terms were included to specify individual anatomical sites (e.g., “oropharyngeal cancer” or “nasopharyngeal cancer”). Only studies published in English were selected for review. No timeframe filters were selected. The search was conducted on October 8, 2021, by one author (N.M.) (Table 1). Identification of current clinical trials was conducted by searching ClinicalTrials.gov using the terms “head and neck cancer” AND “intensity-modulated proton therapy” OR “IMPT”. Trials that were terminated, completed, withdrawn, or have an unknown status were omitted from our selection (7).
Table 1
| Items | Specification |
|---|---|
| Date of search | October 8, 2021 |
| Databases and other sources searched | PubMed |
| Search terms used | “Intensity-modulated proton therapy” OR “IMPT” OR “pencil beam scanning” OR “scanning beam proton therapy” AND “head and neck cancer” |
| Timeframe | No timeframe restrictions were utilized |
| Inclusion and exclusion criteria | Articles reported on clinical trial results in English were included in the study |
| Selection process | Author N.M. conducted the search independently |
| Any additional considerations, if applicable | Additional search terms were included to specify individual anatomical sites |
Treatment planning and delivery
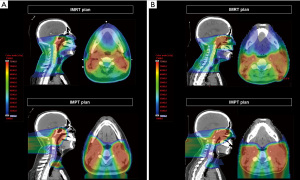
IMPT planning is distinct from IMRT due to the differences in their physical properties. The heavier mass of protons limits the scattering angle, leading to a more defined distribution of the radiation dose. Localizing the Bragg peak to a selected range permits a sharp dose increase to the target with a virtually nonexistent exit dose. Figure 1 illustrates a comparison of IMPT and IMRT plans and the substantial organ sparing achieved in the IMPT plans. Tumors are three-dimensional and heterogeneous, varying in tissue type and thickness; therefore, a spread-out Bragg peak (SOBP) is utilized to cover the entire tumor volume. Significantly, SOBP affects the entrance dose into the skin, leading to skin toxicities that are exacerbated with superficial tumors (8).
Compared to photons, protons are more affected by the tissue it transverses (9). A considerable challenge for planning proton therapy is accounting for variables that shift the location of the Bragg peak. Any shifts in localizing the Bragg peak may lead to insufficient dose to tumor targets and toxicities from irradiating normal tissue. Factors that may impact Bragg peak localization are artifacts (e.g., dental or surgical hardware); air space in cavities (e.g., air-filled sinuses or oral cavity); and patient’s anatomical fluctuations (e.g., tumor shrinkage, weight change, or daily variations in patient positioning). Therefore, treatment plans should consist of short, reliable beam paths that avoid tissue inhomogeneity, such as the mouth, spinal cord, salivary glands, and other critical structures. Furthermore, automated adaptive replanning techniques are integral in considering patient anatomy fluctuations (10-12). Adaptive radiotherapy requires rescanning and replanning during treatment to account for these changes in anatomy. In comparison to IMRT, IMPT is more sensitive to anatomical variations due to the sharp dose drop-off, especially at different tissue types. This requires more frequent assessments to determine the need for adaptive radiotherapy. Cone beam computed tomography (CBCT) is one of the strategies implemented to overcome this limitation in IMPT, where daily positioning and anatomical fluctuations are tracked (10).
There are two primary modes to delivery proton therapy: passive-scatter protons therapy (PSPT) or IMPT. PSPT utilizes scattering foils shaped by customizing the field aperture, proton beamline, and range compensator to target the three-dimensional tumor. IMPT, also known as “pencil-beam scanning” or “active scanning”, is the most recent advancement of delivering proton therapy. IMPT utilizes multiple scanning magnets from different directions that precisely guide the proton beams to target the desired three-dimensional volume. IMPT is more advantageous than PSPT in treating head and neck cancer due to the increased flexibility in targeting complex, irregularly shaped tumors. When planning with IMPT, the patient’s treatment plans can be personalized by either using separate proton beams (single-field optimization) or proton beams synchronously (multiple-field optimization) to target the tumor. Compared to single-field optimization, multiple-filed optimization permits intensity modulation and a higher degree of conformality, allowing for radiation dose deposition at the intended target and not healthy tissue (13). Overall, IMPT has been demonstrated to have dosimetric advantages and fewer adverse effects than PSPT (14,15).
Clinical outcomes and toxicities with IMPT in head and neck cancer
Oropharyngeal cancer (OPC)
Radiation therapy is an integral component of managing OPC in the definitive and adjuvant settings alongside chemotherapy and surgery. IMRT has been successfully used to treat OPC with reduced adverse effects (e.g., dysphagia and xerostomia); however, toxicities remain high in the setting of concurrent chemoradiation (16). As the incidence of HPV-positive OPC increases among younger, healthier populations, it becomes of increased importance to reduce chronic toxicities and improve QoL as patients are expected to live longer after cancer treatment (17). Due to the physical properties of photon therapy, surrounding oropharyngeal and nasopharyngeal healthy tissue are inappropriately radiated. Conversely, proton therapy can be utilized to limit irradiation of normal tissue; thereby, reducing the radiation-related toxicities and improving QoL in OPC patients.
The first prospective trial of OPC patients treated with proton therapy was conducted by Slater et al., where they reported that the 29 patients with locally advanced OPC tolerated photon therapy with concomitant proton boost had an LRC rate of 84% and only three patients (11%) had late grade 3 toxicities (18). Gunn and colleagues reported favorable disease control among the 50 OPC patients (98% HPV+) treated with IMPT. With a median follow-up of 29 months, LRC was 92%, and the most common grade 3 toxicities were acute mucositis (58%) and late dysphagia (12%), and no reported grade 4 or 5 toxicities. Of note, chemotherapy treatment regimes varied considerably (19). In a separate study comparing the same 50 OPC patients, Blanchard and colleagues performed a 2:1 case-matched analysis with 100 patients treated with IMRT and 50 OPC patients treated with IMPT. Both treatment groups had favorable disease control OS and progression-free survival (PFS) with no statistically significant difference. However, IMPT was associated with a significant decrease in severe (grade 3) weight loss at 3-month follow-up (OR 0.44; 95% CI: 0.19–1.0) and 12-month follow-up (OR 0.23; 95% CI: 0.07–0.73) (20). The reduced toxicities noted among OPC patients treated with IMPT are likely due to the reduction in irradiation to the healthy structures located in the oral cavity, salivary glands, larynx, and esophagus (21). An additional 1:1 case-matched analysis comparing 25 IMPT and 25 IMRT OPC patients reported lower radiation doses to surrounding healthy tissue, but additional assessments are required to establish the clinical significance on adverse effects (21).
Aljabab and colleagues conducted a retrospective study of 46 OPC patients treated with IMPT and demonstrated an LRC, PFS, and OS of 100%, 93.5%, and 95.7%, respectively (22). The median follow-up for this study was 19.2 months, so the favorable outcomes should be interpreted with caution. In a study comparing patient-reported outcomes between OPC patients treated with chemotherapy and IMPT (n=35) and IMRT (n=46), IMPT patients had a lower prevalence of changes in taste and appetite (P<0.048). However, it is noteworthy to mention there were statistically significant differences between the clinical T status, induction chemotherapy frequencies, and average radiation dose between the two groups (23). An additional study compared the QoL using prospectively collected patient-reported outcome surveys for OPC patients treated with adjuvant IMPT (n=31) or IMRT (n=33). Patients treated with IMPT had significantly less radiation to normal structures than IMRT, and these dosimetric differences were reflected in the better scores in QoL questionnaires, such as significantly less xerostomia (24). In a comparison of 103 IMPT to 429 IMRT OPC patients, the rates of moderate-severe xerostomia were similar up to 18 months after treatment; however, it became less common in the IMPT group at 18–24 months (6% vs. 20%; P=0.025) and the differences between groups were maintained at 24–36 months (6% vs. 20%; P=0.01) (25).
Reduction in radiation-related adverse events has become increasingly crucial as HPV-related OPC cases increase among younger, healthier patients. These patients have favorable outcomes and are expected to live longer, so reducing chronic toxicities should be emphasized. Clinical trials should further evaluate the efficacy and decrease in adverse events among OPC treated with IMPT. An ongoing randomized clinical study comparing IMRT vs. IMPT in oropharynx cancer is the phase II/III trial NCT01893307 (Table 2). The study aims to compare the cumulative incidence of grade ≥3 toxicities, QoL, OS, and PFS between the two radiotherapies following the treatment of oropharyngeal tumors.
Table 2
| Status | NCT number | Official study title | Locations | Inclusion criteria | Study interventions | Primary endpoints |
|---|---|---|---|---|---|---|
| Recruiting | NCT01893307 | Phase II/III Randomized Trial of Intensity-Modulated Proton Beam Therapy (IMPT) Versus Intensity-Modulated Photon Therapy (IMRT) for the Treatment of Oropharyngeal Cancer of the Head and Neck | MDACC, Mayo Clinic, UF Health, NMH, UMD, MGH, UPHS, UW | SCC of oropharynx (AJCC v7 Stage III–IV) | Randomized to IMRT or IMPT | Rates of late grade 3–5 toxicity between 90 days and 2 years post-RT |
| Recruiting | NCT03164460 | Phase II Randomized Trial of Stereotactic Onco-Ablative Reirradiation Versus Conventionally Fractionated Conformal Radiotherapy for Patients With Small Inoperable Head and Neck Tumors (SOAR-HN) | MDACC | Recurrent HNC or second primary HNC and have previously received at least 30 Gy for HNC | Randomized to SBRT or IMRT/IMPT | 2-year rate of grade 3 or higher toxicity at 2 years post-RT |
| Recruiting | NCT03981068 | DAHANCA 37 A Phase II Study of Intensity Modulated Proton Therapy (IMPT) for Re-irradiation With Curative Intent for Recurrent or New Primary Head and Neck Cancer | Danish Head and Neck Cancer Group | Recurrent HNC or second primary HNC and have previously received RT | Re-irradiation IMPT | Any new grade ≥3 toxicity within 3 years post-RT |
| Recruiting | NCT03513042 | Early Response Evaluation of Proton Therapy by PET-imaging in Squamous Cell Carcinoma Located in the Head and Neck | Holland Proton Therapy Center | Primary unresected invasive HNSCC | IMPT | 3-year local recurrence-free survival |
| Not yet recruiting | NCT05075980 | HEADLIGHT: Hypofractionated Proton Therapy for Head and Neck Cancers | Mayo Clinic | Non-HPV HNC | IMPT | 2-year local/regional failure rate |
Data was acquired from ClinicalTrials.gov, see methods section for details (7). IMPT, intensity-modulated proton therapy; MDACC, MD Anderson Cancer Center; UF Health, University of Florida Health Proton Therapy Institute; NMH, Northwestern Memorial Hospital; UMD, University of Maryland; MGH, Massachusetts General Hospital; UPHS, University of Pennsylvania Health System; UW, University of Washington; SCC, squamous cell carcinoma; AJCC, American Joint Committee of Cancer; IMRT, intensity-modulated radiation therapy; RT, radiation therapy; HNC, head and neck cancer; SBRT, stereotactic body radiation therapy; HPV, human papillomavirus; HNSCC, head and neck squamous cell carcinoma.
Nasopharyngeal cancer
Traditionally, locoregionally advanced nasopharyngeal carcinoma (NPC) is treated with radiation with or without chemotherapy. Radiation delivery to the nasopharynx becomes challenging due to limiting radiation to nearby critical structures, including major salivary glands, pharyngeal constrictors, brain stem, cranial nerves, and spinal cord. Compared to conventional radiotherapy, IMRT has been successfully used to adequately radiate NPCs and decrease the dose to normal tissue, resulting in reduced toxicities and improved QoL (26,27). Although IMRT has been demonstrated to reduce radiation to proximal structures, the nature of photons still leads to increased radiation dose to normal tissue. IMRT has been demonstrated to be inadequate in treating subsets of NPC, such as T4 tumors, Epstein Barr virus (EBV) negative tumors, and locoregional recurrent tumors after initial radiotherapy (28-30). Given the physical properties of proton therapy, IMPT offers an alternative treatment for dose-escalation while sparing nearby healthy tissue.
Chan et al. were the first to report the clinical outcomes of 23 locally advanced NPC patients treated with combined photon and proton therapy in a phase 2 trial. With a median follow-up of 28 months, the patients had favorable outcomes and had disease-free survival (DFS), local control (LC), and OS rates of 90%, 100%, and 100%, respectively. The most common grade 3 toxicities were hearing loss (29%) and weight loss (38%), while no grade 3 xerostomia was observed. Furthermore, no grade 4 or 5 toxicities were reported (31). Holliday and colleagues conducted a 1:2 case-matched analysis comparing 10 IMPT and 20 IMRT NPC patients. They noted a lower prevalence of gastrostomy tube (G-tube) placement among IMPT patients (20% vs. 65%, P=0.02), likely due to the attributed to a lower dose of radiation to the oral cavity. Two patients in the IMRT group and two patients in the IMPT group developed temporal lobe necrosis (TLN) (32). Jiří and colleagues reported low G-tube placement rates (10%) among the 40 NPC patients treated with IMPT. The reported two-year OS, DFS, and LC were 80%, 75%, and 84%, respectively. One patient (2%) developed grade ≥3 TLN (33). At Memorial Sloan Kettering, a retrospective review was completed with 28 IMPT and 49 IMRT patients. IMPT patients developed less grade 2 or higher acute toxicities (OR =0.15, P=0.01). To address potential biases of retrospective studies, a propensity score match of the NPC patients was conducted to balance those treated with IMPT (n=24) and IMRT (n=24). The 2-year PFS in the IMPT and IMRT groups were 95.7% and 76.7% (P=0.14), respectively, and the locoregional failure-free survival rates were 100% and 86.2% (P=0.08), respectively. Grade ≥3 toxicities, such as TLN, osteoradionecrosis, or radiation-induced optic neuropathy were not observed (34).
IMPT is an alternative radiotherapy to treat NPC with favorable treatment outcomes and reductions in toxicities. Prospective clinical trials are essential to confirm and further evaluate the clinical benefits of IMPT in treating NPC patients. Several clinical trials are being conducted to investigate the efficacy of IMPT in treating a variety of head and neck cancer, including NPC (Table 2). More evidence is needed to elucidate whether late toxicities, such as TLN and osteoradionecrosis, are reduced in patients treated with IMPT.
Sinonasal cancer
Most primary sinonasal cancers have favorable outcomes when treated with surgery and adjuvant radiation with or without chemotherapy. In advanced sinonasal disease, surgery may lead to facial disfiguration and neovascular injury due to the complex anatomy involving the paranasal sinuses and nasal cavity and its adjacency to critical organs (35). For unresectable sinonasal tumors, definitive chemoradiation is the preferred treatment. Based on evidence from dosimetry studies, IMPT is superior to IMRT in avoiding radiation to critical structures (36-38). The application of proton therapy in a clinical setting has demonstrated reduced dose to proximal structures and fewer toxicities (39-42).
In one of the most extensive studies supporting proton therapy in head and neck cancer, Patel and colleagues conducted a meta-analysis and systematic review of 41 observational studies, comparing the outcomes of paranasal and nasal cavity cancer patients. From the 43 cohorts included, 286 patients were treated with charged particle therapy, and 1,186 were treated with photon therapy. In the subgroup analysis comparing proton therapy and IMRT, proton therapy had significantly greater LC (RR =1.26, P=0.011) at the longest follow-up and DFS (RR =1.44, P=0.045) at 5-year follow-up. Although potential biases (e.g., selection and publication bias) are inherent when performing a meta-analysis, this study still portrays strong evidence supporting proton therapy in paranasal and nasal cavity cancer patients (43). In a retrospective study at Memorial Sloan Kettering, 86 sinonasal cancer patients were treated with 3D conformal proton technique (3DCPT) (n=40, 47%) or IMPT (n=46, 53%), and outcomes were compared for radiation-naïve and re-radiation cohorts. Compared to 3DCPT, radiation-naïve patients treated with IMPT had significantly better LC (91% versus 72%, P<0.01) (44).
The literature is limited in the long-term outcomes of sinonasal tumors treated with IMPT. The ongoing clinical trials are needed to bolster evidence of IMPT’s ability to improve clinical outcomes and lower toxicities among sinonasal cancer patients (Table 2).
Unilateral head and neck irradiation
Head and neck cancers may be limited to only one side and are not involved with midline structures, such as salivary gland, oral cavity, oropharynx, and skin tumors. IMPT is an excellent radiotherapy alternative to IMRT in unilateral head and neck tumors due to the minimal to no exit dose that spares normal tissue (45).
In a comparison between IMPT and volumetric-modulated arc therapy (VMAT) for treatment of ipsilateral tonsil and salivary gland cancers, IMPT was associated with decreased dose to organs-at-risk and less deterioration in patient-reported outcomes, including pain, swallowing function, dry mouth, sticky saliva, sensory change, cough, speech, feeling ill, and social eating (46). Holliday and colleagues reported on the outcomes of 16 adenoid cystic carcinoma treated with postoperative IMPT. Median follow-up was 24.9 months, and LC was 93.8%. Four patients experienced acute grade 3 toxicities (i.e., dermatitis =3 and oral mucositis =1), and one patient developed a chronic grade 4 optic nerve disorder (47). Zakeri et al. conducted a retrospective review to investigate the outcomes of major salivary gland tumors (MSGTs) treated with proton therapy. Patients were treated with either uniform scanning or IMPT. Overall, MSGTs treated with protons had 3-year LRC, PFS, and OS rates of 95.1% (95% CI: 89.9–100%), 80.7% (95% CI: 70.2–92.7%), and 96.1% (95% CI: 90.9–100%), respectively (48). Another retrospective study evaluating proton therapy in MSGTs was conducted by Chuong and colleagues. Of the 105 patients included, 35 (33.3%) were treated with IMPT, and 70 (66.6%) were treated with uniform scanning. Toxicities were grouped in both radiotherapies, and the acute grade 2 or higher adverse events were nausea (1.5%), dysgeusia (4.8%), xerostomia (7.6%), mucositis (10.5%), and dysphagia (10.5%) (49). Hanania et al. identified 72 MSGT patients—53 (74%) of whom were treated with IMPT—and reported the 2-year LC and OS rates of 96% and 89%, respectively. The most common toxicity grade-3 was radiation dermatitis (21%), with no late-grade ≥3 adverse events were observed (50).
Clinical outcomes with IMPT utilization for unilateral head and neck cancer treatment have limited literature. Most studies cited in this section have included IMPT in their analysis of proton therapy, but minimal data includes the outcomes and toxicities of IMPT in isolation. Further studies are needed to compare IMPT with IMRT to treat unilateral head and neck cancer (Table 2).
Re-irradiation for recurrent head and neck cancer
Some patients treated definitively for head and neck cancer develop disease recurrence, requiring salvage therapy to control the tumor and prevent local, regional death, leading to declines in QoL and eventually death. Salvage therapy may consist of surgical resection followed by re-irradiation or re-irradiation alone. Challenges arise when radioresistant tumors require higher doses of radiation for disease control, but delivering a higher dose is limited by the irradiated healthy tissue from prior treatment. Based on its physical properties, proton therapy has been utilized for recurrent head and neck cancer to escalate radiation dose while avoiding dose overlap to nearby structures, thereby reducing radiation-related toxicities.
In the first multi-institutional clinical retrospective review on proton therapy in recurrent head and neck cancer, Romesser and colleagues identified 92 patients re-irradiated with proton therapy. The proton beam delivery method was uniform scanning beams. Median follow-up was 13.3 months among surviving patients and 10.4 months for all patients. The 1-year rates of locoregional failure, distant metastasis free survival (DMFS), and OS were 25.1%, 84.0%, and 65.2%, respectively. Acute grade toxicities were mucositis (9.9%), dysphagia (9.1%), esophagitis (9.1%), and dermatitis (3.3%). Two patient deaths were reported due to treatment-related bleeding, demonstrating the risk of vascular injury with reirradiation to nearby structures (51).
Compared to other IMRT and other modes of proton delivery, IMPT has limited data on the outcomes of recurrent head and neck cancer treated with re-irradiation due to the novelty of pencil-beam delivery. Phan et al. reported on the outcomes and toxicities of 60 head and neck cancer patients re-irradiated with PSPT (n=15, 25%) or IMPT (n=45, 75%). The median follow-up was 13.6 months, and the 1-year rates of locoregional failure-free survival, OS, PFS, and DMFS were 68.4%, 83.8%, 60.1%, and 74.9%, respectively. Acute grade 3 toxicities were experienced in 18 patients (30%) and 13 patients (22%) needed a feeding tube. The authors reported that three patients may have died from reirradiation-related adverse events (52). In a retrospective review of patients with unresectable, previously irradiated head and neck tumors, 30 patients were re-irradiated with IMPT. The median follow-up was 21 months, and the median OS was 16 months. The 1-year LC, PFS, and OS rates were 52.6%, 21.9%, and 73.4%, respectively, and the 2-year LC, PFS, and OS rates were 21.0%, 10.9%, and 8.4%, respectively. Acute grade 3 toxicity was reported in one patient only, while late severe toxicities were reported in 5 cases (16.6%), including three cases of radiation-induced necrosis, one case of trismus, and one death from carotid bleeding, due to carotid blowout syndrome (53). In a disease-specific retrospective review, previously irradiated, recurrent NPC patients (n=17) treated with IMPT had an 18-month OS and LC rates of 54.4% and 66.6%, respectively. Acute ≥ grade 3 toxicities were not reported. Late ≥ grade 3 toxicities were reported in 23.5% of patients, including hearing impairment (17.6%) as the most frequent (54).
Based on evidence in recent years, IMPT appears to have relatively limited radiation-related toxicities and acceptable outcomes compared to the historical use of photon therapy. However, acute and late adverse events remain frequent, highlighting the ongoing challenge of toxicity in reirradiation in any radiation therapy modality. Vascular and soft tissue complications are a concern for patients re-irradiated and strong considerations should be made in balancing tumor control with the risk of toxicities that may heavily impact morbidity. Prospective clinical trials with IMPT directly compared to other radiation therapy modalities are needed to assess further the dosimetric benefits of IMPT and its improvement on clinical outcomes and toxicities. The Danish Head and Neck Cancer group is conducting a phase II trial, NCT03981068, of re-irradiation with IMPT for recurrent head and neck cancer (Table 2).
Limitations
A limitation in comparing outcomes and toxicities across groups within retrospective trials is the confounding variables that are not controlled. More specifically, variations in the chemotherapy regime between IMPT and IMRT groups may serve as confounders when analyzing patient outcomes. For example, one study had statistically significant differences in induction chemotherapy between the IMPT (74.3%) and the IMRT (23.9%) (23). Therefore, randomized controlled trials are necessary for effectively evaluating differences in IMPT and IMRT.
For more than two decades, proton therapy has been utilized clinically to treat cancer. The significant investment spent in building and operating proton centers have led to higher costs for patients when treated with IMPT compared to IMRT. The limited evidence suggesting IMPT is cost-effective leads to insurance companies rarely providing full coverage, leaving patients with high out-of-pocket costs (55). In a systematic review of proton therapy’s cost-effectiveness, proton therapy was consistently more expensive than photon therapy; however, in some head and neck cancer cohorts, proton therapy exhibited superior cost-effectiveness due to the decrease in expenses for managing toxicities (56). The therapy remains primarily restricted to those who are more affluent. Analysis of the United States National Cancer Database from 2005–2014 revealed patients had an increased likelihood to be treated with proton therapy if they were treated at an academic setting (P<0.001) or were in the highest median household income quartile (>$63,000, P=0.002) (57). Based on this trend, clinicians and health care providers need to ensure patients with limited access to academic centers and patients from lower economic backgrounds will have equitable access to proton therapy to bridge the gap in health disparities. Furthermore, clinical trial recruitment efforts should prioritize these marginalized populations to ensure studies accurately represent patients from all backgrounds. Of note, a study comparing work outcomes in OPC patients randomized to IMPT or IMRT reported increased work and productivity recovery trends, suggesting IMPT may have further financial benefits that need to be explored (58).
Future directions
The use of proton therapy for head and neck cancer appears to have a promising outlook. As the number of proton facilities increases and technological advancements increase efficiency, treatment costs will be reduced. With radiotherapy becoming less expensive, more patients will receive proton therapy, and more clinical research can be conducted to evaluate the favorable outcomes further. Improvements in automating proton plan adoption and on-board imaging will facilitate an efficient system (59). Furthermore, a decrease in treatment-related toxicities will reduce expenses on managing toxicities.
Randomized clinical trials are necessary to comprehensively analyze the benefits of using proton therapy compared to photon therapy. More specifically, trials can directly compare IMPT with other radiation delivery methods, such as IMRT. Ongoing trials are investigating optimal dose, delivery method, and patient populations that will result in favorable clinical outcomes and reduce adverse events to improve QoL (Table 2). Evidence from these studies will promote an appropriate adoption of proton therapy in treating head and neck cancer. While proton therapy becomes widely utilized, additional research should explore protons’ interactions with immunotherapy (60). Interactions between proton therapy and immunotherapy should be investigated to understand and potentially enhance the immunologic response to cancer cells. Additionally, research on proton therapy’s effect on biological mechanisms should be considered. It has been reported that the expression of RNA and proteins involved in angiogenesis, inflammation, proliferation, and anti-tumor immune responses varies in response to proton radiation (61,62). These findings may clarify the improved clinical outcomes with proton therapy compared to photons, and this information can strengthen synergistic effects with other treatment modalities, including anti-angiogenic and anti-immune checkpoint inhibitors (61).
Evidence from clinical trials may take years to enhance standard of care; therefore, models are being developed to predict the benefits, risks, and cost-effectiveness in selecting proton versus photon radiation (63-65). Normal tissue complication probability (NTCP) models have been developed to predict the risk of toxicities (66,67). For example, one study evaluated the implementation of model-based selection in treating head and neck cancer with IMPT and reported higher IMPT selection rates among patients with advanced disease and a significant decrease in organs at risk doses compared to VMAT (68). The development and improvement in these models will assist in making personalized treatment decisions and appropriately select patients who will benefit from IMPT over IMRT.
Conclusions
IMPT offers a dosimetric advantage over IMRT due to the Bragg peak phenomenon, where the proton dose distribution is maximized at the targeted tumor, sparing nearby, healthy tissue. Based on the outcomes and toxicities discussed in this review, IMPT is a promising head and neck cancer therapy. While there are some challenges to implementing IMPT, such as the shifts in localizing the Bragg peak due to artifacts or anatomical fluctuations, technological advancements will limit these uncertainties and allow for broader adoption of the radiotherapy. As the number of patients receiving IMPT increases, we can further evaluate the favorable clinical outcomes and limited toxicities in the chronic setting. As ongoing prospective trials will elucidate the acute and long-term benefits, clinicians and healthcare providers should use evidence-based medicine to optimize treatment to improve the health and QoL of our head and neck cancer patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Therapeutic Radiology and Oncology for the series “Pencil Beam Scanning Particle Therapy”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tro.amegroups.com/article/view/10.21037/tro-22-8/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (Available at https://tro.amegroups.com/article/view/10.21037/tro-22-8/coif). The series “Pencil Beam Scanning Particle Therapy” was commissioned by the editorial office without any funding or sponsorship. NYL served as the unpaid Guest Editor of the series. NYL disclosed relationships with Merck & Co, Merck EMD, Mirati Therapeutics, and Elsie Pharmaceuticals. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pfister DG, Spencer S, Adelstein D, et al. Head and Neck Cancers, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2020;18:873-98. [Crossref] [PubMed]
- Rosenthal DI, Chambers MS, Fuller CD, et al. Beam path toxicities to non-target structures during intensity-modulated radiation therapy for head and neck cancer. Int J Radiat Oncol Biol Phys 2008;72:747-55. [Crossref] [PubMed]
- Endo M, Robert R. Wilson (1914-2000): the first scientist to propose particle therapy-use of particle beam for cancer treatment. Radiol Phys Technol 2018;11:1-6. [Crossref] [PubMed]
- LAWRENCE JH. Pituitary irradiation with high-energy proton beams: a preliminary report. Cancer Res 1958;18:121-34. [PubMed]
- WILSON RR. Radiological use of fast protons. Radiology 1946;47:487-91. [Crossref] [PubMed]
- Kooy HM, Grassberger C. Intensity modulated proton therapy. Br J Radiol 2015;88:20150195. [Crossref] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=Head+and+Neck+Cancer&term=%22intensity-modulated+proton+therapy%22+OR+%22IMPT%22&cntry=&state=&city=&dist=. Accessed October 16, 2021.
- Leeman JE, Romesser PB, Zhou Y, et al. Proton therapy for head and neck cancer: expanding the therapeutic window. Lancet Oncol 2017;18:e254-65. [Crossref] [PubMed]
- Mohan R, Grosshans D. Proton therapy - Present and future. Adv Drug Deliv Rev 2017;109:26-44. [Crossref] [PubMed]
- De Ornelas M, Xu Y, Padgett K, et al. CBCT-Based Adaptive Assessment Workflow for Intensity Modulated Proton Therapy for Head and Neck Cancer. Int J Part Ther 2021;7:29-41. [Crossref] [PubMed]
- Wu RY, Liu AY, Sio TT, et al. Intensity-Modulated Proton Therapy Adaptive Planning for Patients with Oropharyngeal Cancer. Int J Part Ther 2017;4:26-34. [Crossref] [PubMed]
- Yang Z, Zhang X, Wang X, et al. Multiple-CT optimization: An adaptive optimization method to account for anatomical changes in intensity-modulated proton therapy for head and neck cancers. Radiother Oncol 2020;142:124-32. [Crossref] [PubMed]
- Shen J, Liu W, Anand A, et al. Impact of range shifter material on proton pencil beam spot characteristics. Med Phys 2015;42:1335-40. [Crossref] [PubMed]
- Ding X, Li X, Qin A, et al. Redefine the role of range shifter in treating bilateral head and neck cancer in the era of Intensity Modulated Proton Therapy. J Appl Clin Med Phys 2018;19:749-55. [Crossref] [PubMed]
- Frank SJ, Cox JD, Gillin M, et al. Multifield optimization intensity modulated proton therapy for head and neck tumors: a translation to practice. Int J Radiat Oncol Biol Phys 2014;89:846-53. [Crossref] [PubMed]
- Nguyen-Tan PF, Zhang Q, Ang KK, et al. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: long-term report of efficacy and toxicity. J Clin Oncol 2014;32:3858-66. [Crossref] [PubMed]
- Jemal A, Simard EP, Dorell C, et al. Annual Report to the Nation on the Status of Cancer, 1975-2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst 2013;105:175-201. [Crossref] [PubMed]
- Slater JD, Yonemoto LT, Mantik DW, et al. Proton radiation for treatment of cancer of the oropharynx: early experience at Loma Linda University Medical Center using a concomitant boost technique. Int J Radiat Oncol Biol Phys 2005;62:494-500. [Crossref] [PubMed]
- Gunn GB, Blanchard P, Garden AS, et al. Clinical Outcomes and Patterns of Disease Recurrence After Intensity Modulated Proton Therapy for Oropharyngeal Squamous Carcinoma. Int J Radiat Oncol Biol Phys 2016;95:360-7. [Crossref] [PubMed]
- Blanchard P, Garden AS, Gunn GB, et al. Intensity-modulated proton beam therapy (IMPT) versus intensity-modulated photon therapy (IMRT) for patients with oropharynx cancer - A case matched analysis. Radiother Oncol 2016;120:48-55. [Crossref] [PubMed]
- Holliday EB, Kocak-Uzel E, Feng L, et al. Dosimetric advantages of intensity-modulated proton therapy for oropharyngeal cancer compared with intensity-modulated radiation: A case-matched control analysis. Med Dosim 2016;41:189-94. [Crossref] [PubMed]
- Aljabab S, Liu A, Wong T, et al. Proton Therapy for Locally Advanced Oropharyngeal Cancer: Initial Clinical Experience at the University of Washington. Int J Part Ther 2020;6:1-12. [Crossref] [PubMed]
- Sio TT, Lin HK, Shi Q, et al. Intensity Modulated Proton Therapy Versus Intensity Modulated Photon Radiation Therapy for Oropharyngeal Cancer: First Comparative Results of Patient-Reported Outcomes. Int J Radiat Oncol Biol Phys 2016;95:1107-14. [Crossref] [PubMed]
- Sharma S, Zhou O, Thompson R, et al. Quality of Life of Postoperative Photon versus Proton Radiation Therapy for Oropharynx Cancer. Int J Part Ther 2018;5:11-7. [Crossref] [PubMed]
- Cao J, Zhang X, Jiang B, et al. Intensity-modulated proton therapy for oropharyngeal cancer reduces rates of late xerostomia. Radiother Oncol 2021;160:32-9. [Crossref] [PubMed]
- Kuang WL, Zhou Q, Shen LF. Outcomes and prognostic factors of conformal radiotherapy versus intensity-modulated radiotherapy for nasopharyngeal carcinoma. Clin Transl Oncol 2012;14:783-90. [Crossref] [PubMed]
- Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol 2011;12:127-36. [Crossref] [PubMed]
- Han F, Zhao C, Huang SM, et al. Long-term outcomes and prognostic factors of re-irradiation for locally recurrent nasopharyngeal carcinoma using intensity-modulated radiotherapy. Clin Oncol (R Coll Radiol) 2012;24:569-76. [Crossref] [PubMed]
- Huang HI, Chan KT, Shu CH, et al. T4-locally advanced nasopharyngeal carcinoma: prognostic influence of cranial nerve involvement in different radiotherapy techniques. ScientificWorldJournal 2013;2013:439073. [Crossref] [PubMed]
- Stenmark MH, McHugh JB, Schipper M, et al. Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys 2014;88:580-8. [Crossref] [PubMed]
- Chan A, Adams J, Weyman E, et al. A Phase II Trial of Proton Radiation Therapy With Chemotherapy for Nasopharyngeal Carcinoma. Int J Radiat Oncol Biol Phys 2012;84:S151-2. [Crossref]
- Holliday EB, Garden AS, Rosenthal DI, et al. Proton Therapy Reduces Treatment-Related Toxicities for Patients with Nasopharyngeal Cancer: A Case-Match Control Study of Intensity-Modulated Proton Therapy and Intensity-Modulated Photon Therapy. Int J Part Ther 2015;2:19-28. [Crossref]
- Jiří K, Vladimír V, Michal A, et al. Proton pencil-beam scanning radiotherapy in the treatment of nasopharyngeal cancer: dosimetric parameters and 2-year results. Eur Arch Otorhinolaryngol 2021;278:763-9. [Crossref] [PubMed]
- Li X, Kitpanit S, Lee A, et al. Toxicity Profiles and Survival Outcomes Among Patients With Nonmetastatic Nasopharyngeal Carcinoma Treated With Intensity-Modulated Proton Therapy vs Intensity-Modulated Radiation Therapy. JAMA Netw Open 2021;4:e2113205. [Crossref] [PubMed]
- Waldron JN, O'Sullivan B, Warde P, et al. Ethmoid sinus cancer: twenty-nine cases managed with primary radiation therapy. Int J Radiat Oncol Biol Phys 1998;41:361-9. [Crossref] [PubMed]
- Chera BS, Malyapa R, Louis D, et al. Proton therapy for maxillary sinus carcinoma. Am J Clin Oncol 2009;32:296-303. [Crossref] [PubMed]
- Lomax AJ, Goitein M, Adams J. Intensity modulation in radiotherapy: photons versus protons in the paranasal sinus. Radiother Oncol 2003;66:11-8. [Crossref] [PubMed]
- Mock U, Georg D, Bogner J, et al. Treatment planning comparison of conventional, 3D conformal, and intensity-modulated photon (IMRT) and proton therapy for paranasal sinus carcinoma. Int J Radiat Oncol Biol Phys 2004;58:147-54. [Crossref] [PubMed]
- Fitzek MM, Thornton AF, Varvares M, et al. Neuroendocrine tumors of the sinonasal tract. Results of a prospective study incorporating chemotherapy, surgery, and combined proton-photon radiotherapy. Cancer 2002;94:2623-34. [Crossref] [PubMed]
- Nakamura N, Zenda S, Tahara M, et al. Proton beam therapy for olfactory neuroblastoma. Radiother Oncol 2017;122:368-72. [Crossref] [PubMed]
- Pommier P, Liebsch NJ, Deschler DG, et al. Proton beam radiation therapy for skull base adenoid cystic carcinoma. Arch Otolaryngol Head Neck Surg 2006;132:1242-9. [Crossref] [PubMed]
- Truong MT, Kamat UR, Liebsch NJ, et al. Proton radiation therapy for primary sphenoid sinus malignancies: treatment outcome and prognostic factors. Head Neck 2009;31:1297-308. [Crossref] [PubMed]
- Patel SH, Wang Z, Wong WW, et al. Charged particle therapy versus photon therapy for paranasal sinus and nasal cavity malignant diseases: a systematic review and meta-analysis. Lancet Oncol 2014;15:1027-38. [Crossref] [PubMed]
- Fan M, Kang JJ, Lee A, et al. Outcomes and toxicities of definitive radiotherapy and reirradiation using 3-dimensional conformal or intensity-modulated (pencil beam) proton therapy for patients with nasal cavity and paranasal sinus malignancies. Cancer 2020;126:1905-16. [Crossref] [PubMed]
- Kandula S, Zhu X, Garden AS, et al. Spot-scanning beam proton therapy vs intensity-modulated radiation therapy for ipsilateral head and neck malignancies: a treatment planning comparison. Med Dosim 2013;38:390-4. [Crossref] [PubMed]
- Jeans EB, Shiraishi S, Manzar G, et al. An comparison of acute toxicities and patient-reported outcomes between intensity-modulated proton therapy and volumetric-modulated arc therapy after ipsilateral radiation for head and neck cancers. Head Neck 2022;44:359-71. [Crossref] [PubMed]
- Holliday E, Bhattasali O, Kies MS, et al. Postoperative Intensity-Modulated Proton Therapy for Head and Neck Adenoid Cystic Carcinoma. Int J Part Ther 2016;2:533-43. [Crossref] [PubMed]
- Zakeri K, Wang H, Kang JJ, et al. Outcomes and prognostic factors of major salivary gland tumors treated with proton beam radiation therapy. Head Neck 2021;43:1056-62. [Crossref] [PubMed]
- Chuong M, Bryant J, Hartsell W, et al. Minimal acute toxicity from proton beam therapy for major salivary gland cancer. Acta Oncol 2020;59:196-200. [Crossref] [PubMed]
- Hanania AN, Zhang X, Gunn GB, et al. Proton Therapy for Major Salivary Gland Cancer: Clinical Outcomes. Int J Part Ther 2021;8:261-72. [Crossref] [PubMed]
- Romesser PB, Cahlon O, Scher ED, et al. Proton Beam Reirradiation for Recurrent Head and Neck Cancer: Multi-institutional Report on Feasibility and Early Outcomes. Int J Radiat Oncol Biol Phys 2016;95:386-95. [Crossref] [PubMed]
- Phan J, Sio TT, Nguyen TP, et al. Reirradiation of Head and Neck Cancers With Proton Therapy: Outcomes and Analyses. Int J Radiat Oncol Biol Phys 2016;96:30-41. [Crossref] [PubMed]
- Gordon K, Gulidov I, Semenov A, et al. Proton re-irradiation of unresectable recurrent head and neck cancers. Rep Pract Oncol Radiother 2021;26:203-10. [Crossref] [PubMed]
- Dionisi F, Croci S, Giacomelli I, et al. Clinical results of proton therapy reirradiation for recurrent nasopharyngeal carcinoma. Acta Oncol 2019;58:1238-45. [Crossref] [PubMed]
- Ramaekers BL, Grutters JP, Pijls-Johannesma M, et al. Protons in head-and-neck cancer: bridging the gap of evidence. Int J Radiat Oncol Biol Phys 2013;85:1282-8. [Crossref] [PubMed]
- Verma V, Mishra MV, Mehta MP. A systematic review of the cost and cost-effectiveness studies of proton radiotherapy. Cancer 2016;122:1483-501. [Crossref] [PubMed]
- Lee A, Kang J, Yu Y, et al. Trends and Disparities of Proton Therapy Use among Patients with Head and Neck Cancer: Analysis from the National Cancer Database (2005-14). Int J Part Ther 2019;5:1-10. [Crossref] [PubMed]
- Smith GL, Fu S, Ning MS, et al. Work Outcomes after Intensity-Modulated Proton Therapy (IMPT) versus Intensity-Modulated Photon Therapy (IMRT) for Oropharyngeal Cancer. Int J Part Ther 2021;8:319-27. [Crossref] [PubMed]
- Kurz C, Nijhuis R, Reiner M, et al. Feasibility of automated proton therapy plan adaptation for head and neck tumors using cone beam CT images. Radiat Oncol 2016;11:64. [Crossref] [PubMed]
- Gameiro SR, Malamas AS, Bernstein MB, et al. Tumor Cells Surviving Exposure to Proton or Photon Radiation Share a Common Immunogenic Modulation Signature, Rendering Them More Sensitive to T Cell-Mediated Killing. Int J Radiat Oncol Biol Phys 2016;95:120-30. [Crossref] [PubMed]
- Lupu-Plesu M, Claren A, Martial S, et al. Effects of proton versus photon irradiation on (lymph)angiogenic, inflammatory, proliferative and anti-tumor immune responses in head and neck squamous cell carcinoma. Oncogenesis 2017;6:e354. [Crossref] [PubMed]
- Wang L, Yang L, Han S, et al. Patterns of protein expression in human head and neck cancer cell lines differ after proton vs photon radiotherapy. Head Neck 2020;42:289-301. [Crossref] [PubMed]
- Brodin NP, Kabarriti R, Pankuch M, et al. A Quantitative Clinical Decision-Support Strategy Identifying Which Patients With Oropharyngeal Head and Neck Cancer May Benefit the Most From Proton Radiation Therapy. Int J Radiat Oncol Biol Phys 2019;104:540-52. [Crossref] [PubMed]
- Quik EH, Feenstra TL, Postmus D, et al. Individual patient information to select patients for different radiation techniques. Eur J Cancer 2016;62:18-27. [Crossref] [PubMed]
- Lühr A, Löck S, Roth K, et al. Concept for individualized patient allocation: ReCompare--remote comparison of particle and photon treatment plans. Radiat Oncol 2014;9:59. [Crossref] [PubMed]
- Langendijk JA, Lambin P, De Ruysscher D, et al. Selection of patients for radiotherapy with protons aiming at reduction of side effects: the model-based approach. Radiother Oncol 2013;107:267-73. [Crossref] [PubMed]
- Jakobi A, Stützer K, Bandurska-Luque A, et al. NTCP reduction for advanced head and neck cancer patients using proton therapy for complete or sequential boost treatment versus photon therapy. Acta Oncol 2015;54:1658-64. [Crossref] [PubMed]
- Tambas M, Steenbakkers RJHM, van der Laan HP, et al. First experience with model-based selection of head and neck cancer patients for proton therapy. Radiother Oncol 2020;151:206-13. [Crossref] [PubMed]
Cite this article as: Mohamed N, Li X, Lee NY. A narrative review of intensity-modulated proton therapy for head and neck cancer. Ther Radiol Oncol 2023;7:4.


