
Absolute dose measurement and energy dependence of LiF dosimeters in proton therapy beam dosimetry
Introduction
In recent decades, ion beam radiotherapy has become an increasingly popular method of cancer treatment due to its dosimetric advantages to achieve desired outcomes and is arguably superior to photon or electron treatments in the clinical setting (1,2). To implant the proton therapy, dosimetric commission and verification are important fundamental before patient treatment with kinds of active and passive detectors. An ionization chamber is a gold dosimetric standard tool. Other dosimetric tools such as radiochromic film, thermoluminescent dosimeter (TLD), optically stimulated luminescence dosimeter (OSLD), metal oxide-semiconductor field-effect transistor (MOSFET) detector, and radiophotoluminescent glass dosimeter (RGD) are also used in particle dosimetry. According to the studies, the feature of linear energy transfer (LET) dependence is a notable concern because of the influence on dosimetry. EBT3 film under-response increased with dose-averaged LET (LETd), from approximately 10% under-response for LETd =5 keV/µm to approximately 20% for LETd =8 keV/µm. With correction, the corrected film profile was within 2% and 1 mm of the Monte Carlo profile (3). The magnitude of the LET dependence of RGD increased with LET; for an LET of 8.2 keV/µm, the RGD under-response was up to 16%. The LET-corrected RGD dose was within 5% of the corresponding ionization chamber dose at all energies until 200 MeV, where it was 5.3% lower than the ionization chamber dose (4). TLDs are small changed in LET and the dose measurements in a proton beam were accurate to within 5.0% of the expected dose (5). Moreover, TLDs and OSLDs exhibit an over-response and an under-response of 7% and 4%, respectively (6).
TLDs are widely used in radiation therapy as a dosimetric tool and for personal radiation monitoring (5,7,8), as TLDs are composed of tissue equivalent materials, small in size, and effectively report dose points of interest for dose verification and in vivo dosimetry (9,10). Many types of TLDs have been developed for different applications, including those with higher sensitivity materials for low-dose measurements, those composed of different materials with various interaction mechanisms for low- or high-density radiation, and those with a wide dose range for unknown space environments (11). Studies have investigated the effectiveness of TLDs for use in ion-beam dosimetry, and for determining the average LET of protons or other heavy charged particles by the relative efficiency and high temperature ratio (HTR) method (12-14). The relative efficiency, a parameter which depends on ionization density, can be an effective indicator to evaluate the relationship between particle energy and thermoluminescent (TL) response (15,16). Meanwhile, the HTR method is based on the enhanced relative intensity of the high-temperature region in the glow curve following high-LET irradiation (17-19). For dosimetric purposes in proton therapy, TLDs can be used for in vivo dose measurement. The LET dependence of TLDs may perturb particle beam dosimetry without corrections, particularly for high LET beams (6). The influence of LET for ion beam dosimetry must be considered carefully. The LET of ion beams depends on the energy and types of particles, including proton, helium, and carbon. In clinical applications of proton radiotherapy, TLD dosimetry may experience an unknown energy spectrum at the point of interest, as proton energy is related to LET, and it is thus challenging to predict the subsequent influence on dosimetry, thereby causing dosimetric measurement uncertainty. The aim of this study is to evaluate the TL response in different proton energy spectrums by determining the relative efficiency and the establish the influence tables on proton beam dosimetry.
Methods
TLD dosimetry system
Two types of TLD chips were used in this study, LiF: Mg, Ti (TLD-100TM, Thermo Fisher Scientific, OH, USA) with dimensions of 3.1×3.1×0.89 mm3, and LiF: Mg, Cu, P (MCP-100, RadPro International GmbH, Germany) with dimensions of 3.2×3.2×0.9 mm3. Two stages of the annealing procedure were performed, before and after the readout. The annealing procedure before the readout consisted of 10 minutes at 100 ℃ for both the TLD-100 and MCP-100. The purpose was to minimize the uncertainty arising from the unstable signal of the low-temperature regions which are more sensitive to fading (11) and easily influenced by vibration and temperature, potentially increasing the uncertainty of the glow curve signal. The annealing procedures after the readout consisted of 1 hour at 400 ℃ then 2 hours at 100 ℃ followed by natural cooling to room temperature for the TLD-100, and 20 minutes at 250 ℃ followed by rapid cooling to room temperature for MCP-100. The purpose was to remove the residual signal in the deep trap and to stabilize the lattice of the TL crystals (12). The cooling rate of the annealing procedure has a considerable effect on the supra-linearity and relative efficiency after proton irradiation (13). The time schedules were used to control the time of each stage of the annealing procedure to minimize the uncertainties associated with temperature and time.
The readout system used in the investigation was a manual Harshaw Series 3500 (Thermo Fisher Scientific, OH, USA). Before readout, the reader was warmed up for at least 30 minutes. The TL glow curves were obtained by heating the TLD chips from 50 to 400 ℃ for the TLD-100, and from 50 to 240 ℃ for the MCP-100 at a constant heating rate of 5 ℃/s. A proper flux of nitrogen flowed throughout the reading session. The TLDs were read out within 24 hours after irradiation to minimize the fading effect. The TL response was quantified by total integration of the glow curve for both the TLD-100 and MCP-100 in this study. This method applied was relatively simple, as the TL glow curves of LiF types consist of several overlapped peaks (15); however, this method is more stable and convenient for use in clinical settings. The TLD-100 with a reproducibility of 3% and MCP-100 with a reproducibility of 5% were selected by using the linear accelerator operated at 6 MV photon beam, irradiated at the same dose with the same set-up conditions three times. As the MCP-100 is highly sensitive with higher uncertainty, the reproducibility of the MCP-100 was set at 5% for clinical usage. In order to minimize the different sensitivities between the TLD chips, individual response factors for each dosimeter were determined (6).
Dose calibration and validation
The photon dose response curve was determined using a 6-MV photon beam with linear accelerator (Clinac iXTM, Varian, CA, USA). The TLD chips were placed at a depth of 5 cm in the solid water phantom (Figure 1A). The proton dose response curve was determined using a 190 MeV proton beam with wobbling nozzle (Sumitomo Heavy Industry, Japan), which was generated a uniform dose by rotating a pencil beam with x and y magnets and passing through a scatter, and MLC openings of 10×10 cm2. The TLD chips were placed at a depth of 13.8 cm in high-density polyethylene (HDPE) phantom (Figure 1B), that is, the center of 10-cm spread-out Bragg peak (SOBP), which was generated by ridge filter. The dose calibration curve was ranged from 25 to 500 cGy. Each point of measurement used 5 TLD chips, while the standard deviation shows the discrepancies between these 5 TLDs. The absolute dose was determined with a PTW 30013 waterproof Farmer ionization chamber (PTW-Freiburg GmbH, Freiburg, Germany) placed at the same depth as the irradiated TLDs.

To validate the photon dose calibration, TLDs were placed at a depth of 5 cm in the solid water phantom and the irradiated dose is differed from the dose point of calibration. To validate the proton dose calibration, the depth dose curve of 230 MeV pristine pencil beam with wobbling nozzle was determined by irradiating TLDs in HDPE phantom at various depths (Figure 1C). All TLD measurement results were compared with ionization chamber.
Relative efficiency
Relative efficiency is a quantification to realize the different response of TLDs irradiated using different radiation types at an unit of physical dose, due to the different energies deposited in the TLDs (20). The relative efficiency is the TL response per unit physical dose produced by any radiation, with respect to the TL response per unit physical dose produced by a reference radiation:
where R(p) and R(γ) are the TL response for the radiation under study (proton beam) and the reference radiation (6 MV X-ray) at dose levels D(p) and D(γ), respectively. The dependence of the relative efficiency on proton energy was investigated for both types of TLDs. The TLD chips were placed at a depth of 2 cm in HDPE phantom and irradiated with the nominal proton beam energy varying from 70 to 230 MeV. Additionally, the lower proton energy (<70 MeV) was performed at various deep depths along a pristine Bragg peak of 230 MeV. The proton energy spectrum at the irradiation position was calculated by a Geant4-based (GEometry And Tracking-version 4, version 9.1. Patch02) Monte Carlo simulation platform, the particle therapy simulation framework (PT-Sim, 2014 released), carried out with a validated beamline model including all the components in a proton therapy nozzle at the Linkuo Chang Gung Memorial Hospital (21,22). The energy spectrums were simulated in a cubic water phantom (30×30×30 cm3) at all depths that TLD chips irradiated with a scoring size of 3×3×0.09 cm3, the same thickness as TLD chips. The energy spectrums were analyzed by Matlab (2017b, MathWorks, USA) and the mean energy of each proton spectrum was calculated.
Results
Dose calibration and validation
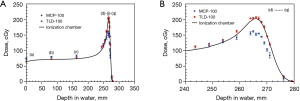
For the photon and proton dose calibration curves, a second-order polynomial was fitted to the relation of dose and TL response with R2 of 0.999. For photon dose validation, the measurement dose difference between TLDs and ionization chamber is smaller than 2.3% for TLD-100 and 2.5% for MCP-100. For proton dose validation, the measurement dose difference between TLD-100 and ionization chamber was −1.1% to 8.1%, while MCP-100 was −27.7% to 13% without energy dependence correction (Figure 2A). The larger dose discrepancy was at the region of Bragg peak (Figure 2B).
Relative efficiency
Figure 3 shows the relationship between the relative efficiency and proton mean energy, as calculated from the energy spectrum at the irradiation position (Figure 4A,4B). The mean proton energy of 83.5 MeV was the cut-off energy for different trends of relative efficiency. The relative efficiency of TLD-100 ranged from 0.95 to 0.982 with a mean proton energy of 215.8 to 83.5 MeV, and a slight influence of proton energy dependence observed. The influence of the proton energy at this energy region had approximately 3.2% dose uncertainty. For the mean proton energy between 83.5 and 30.5 MeV, the relative efficiency increased from 0.982 to 1.13; meanwhile, below 30.5 MeV, it dropped dramatically. The relative efficiency of MCP-100 ranged from 0.810 to 0.749 with a mean proton energy of 215.8 to 83.5 MeV. The influence of the proton energy at this region had approximately 6.1% dose uncertainty; of note, at lower than 83.5 MeV, the relative efficiency continuously decreases along with decreasing energy.
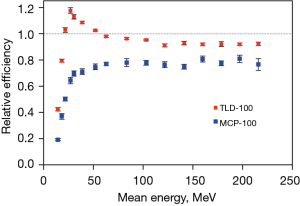
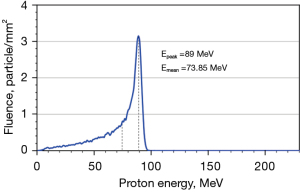
There are two examples of energy dependence correction. In Figure 2B, the mean energy of dose point (e) is 54.7 MeV obtained from simulation. The energy spectrums of proton calibration condition were simulated and shown in Figure 5, contained a portion of low energy proton and dominant energy of 89 MeV with a mean energy of 73.85 MeV. Derived from Figure 3, the relative efficiency of TLD-100 and MCP-100 was 0.91 and 0.77, respectively. For TLD-100, the relative efficiency ratio of dose point (e) to calibration condition was 1.06 and the dose of TLD-100 was 7.9% higher than ionization chamber. Appling the energy-dependence correction, the dose difference between TLD-100 and ionization chamber is 1.9%. For MCP-100, the relative efficiency ratio of dose point (e) to calibration condition is 0.99 and the dose of MCP-100 is 4.7% higher than ionization chamber. Appling the energy dependence correction, the dose difference between TLD-100 and ionization chamber was 4.75%. Another example is dose point (h) and its mean energy was 24.6 MeV obtained from simulation. For TLD-100, the relative efficiency ratio of dose point (h) to calibration condition was 1.23 and the dose of TLD-100 was 10.6% higher than ionization chamber. Appling the energy-dependence correction, the dose difference between TLD-100 and ionization chamber is −11.7%. For MCP-100, the relative efficiency ratio of dose point (g) to calibration condition was 0.89 and the dose of MCP-100 was 20.8% lower than ionization chamber. Appling the energy dependence correction, the dose difference between MCP-100 and ionization chamber was −9.2%.
Uncertainty evaluation
Tables 1,2 list the sources of uncertainty and the estimated values of their magnitudes for the TLD-100 and MCP-100 with X-ray beam and proton beam. The overall uncertainty is using error propagation to calculate the total uncertainty of TL dosimetry in this study. TLD-100 has a photon dose uncertainty of 3.78%, and proton dose uncertainty of 4.67% and 15.18% for high energy and low energy proton beams, respectively. MCP-100 has a photon dose uncertainty of 5.59%, and proton dose uncertainty of 8.16% and 28.52% for high energy and low energy proton beams, respectively. High and low proton energies were separated with a cutoff energy of 80 MeV.
Table 1
| Source of uncertainty | Uncertainty (%)— photon |
Uncertainty (%)— high E proton1 |
Uncertainty (%)— low E proton2 |
|---|---|---|---|
| Reproducibility | 3 | 3 | 3 |
| Dose delivered by linear accelerator | 0.1 | – | – |
| Dose delivered by cyclotron | – | 0.1 | 0.1 |
| Determination of dose calibration curve3 | 2.3 | 1.8 | 1.8 |
| Energy dependence for proton | – | 3.2 | 14.1 |
| Fading correction | – | – | – |
| Directional dependence4 | – | – | – |
| Overall uncertainty | 3.78 | 4.67 | 15.18 |
1, high E proton means the mean or maximum proton energy of the spectrum higher than 80 MeV; 2, lower E proton means the mean or maximum proton energy of the spectrum higher than 30 MeV and lower than 80 MeV; 3, dose is range from 25 to 500 cGy; 4, in this study, all the experiments used the perpendicular direction radiation beam. TLD, thermoluminescent dosimeter.
Table 2
| Source of uncertainty | Uncertainty (%)— photon |
Uncertainty (%)— high E proton1 |
Uncertainty (%)— low E proton2 |
|---|---|---|---|
| Reproducibility | 5 | 5 | 5 |
| Dose delivered by linear accelerator | 0.1 | – | – |
| Dose delivered by cyclotron | – | 0.1 | 0.1 |
| Determination of dose calibration curve3 | 2.5 | 2.1 | 2.1 |
| Energy dependence for proton | – | 6.1 | 28 |
| Fading correction | – | – | – |
| Directional dependence4 | – | – | – |
| Overall uncertainty | 5.59 | 8.16 | 28.52 |
1, high E proton means the mean or maximum proton energy of the spectrum higher than 80 MeV; 2, lower E proton means the mean or maximum proton energy of the spectrum higher than 30 MeV and lower than 80 MeV; 3, dose is range from 25 to 500 cGy; 4, in this study, all the experiments used the perpendicular direction radiation beam.
Discussion
Photon energy in treatment field don’t change too much and the photon energy dependence of TLD is relatively small, so it can be ignored within an acceptable dose uncertainty; however, proton energy in the treatment field, proton energy would affect TL response (17,23,24), that is, the same physical dose deposited from different proton energy would obtain different TL response. In this study, a percentage depth dose (PDD) measurement using TLDs and ionization chamber for comparison was demonstrated and it is a good way to validate the dose calibration of TLDs and observe how energy dependence affects dosimetry, because proton energy is decreased as depths increased. The results of the dose measurements at various depths using TLDs are shown in Figure 2A, as compared with an ionization chamber. TLD-100 demonstrated a closer correlation to the ionization chamber, and the larger dose discrepancy showed at the Bragg peak region. MCP-100 is higher than that of the ionization chamber at shallower depths, though trending lower than the ionization chamber at the Bragg peak region. The sources of dose uncertainty were summarized below: (I) energy dependence of TLD reveal as relative efficiency; (II) experiment set-up uncertainty; (III) partial volume effect of TLD (0.89 mm thickness of TLD chips); (IV) the intrinsic reproducibility uncertainty of TLD; (V) energy spectrum of dose calibration condition; (VI) glow curve analysis method.
Without consideration of proton energy dependence, the dose calculation uncertainty increased, especially with a lower proton energy. In clinical applications, the point of interest has a wide-ranging proton energy spectrum in a mixed radiation field, it is thus challenging to determine the influence on the TL response even using energy-dependence correction factor. The key influences on the proton dose measurement of TLD is the conditions of calibration, including the proton energy spectrum for dose calibration, and the method used for glow curve analysis. The dose measurement results would be different as the calibration condition or glow curve analysis method changed. In this study, we used total integration method to get TL response. Glow curve analysis can present the properties of TLDs (6,25). No significant difference was noted between the photon and proton for the TLD-100, even at the high temperature region. For the MCP-100, the main peak height of the proton was less than that of the photon, and the area of the high temperature region was greater than that of the photon as the proton energy decreased (25)
Several studies have demonstrated that TLD-100 has the potential to evaluate proton energy, which is related to LET and the relative biological effectiveness (RBE) (12). The TL response at high-temperature region of the glow curve increased, as the ionization density of the particle correlates to the energy deposition at the TLDs increased (23). Meanwhile, the HTR method, which is a parameter quantifying the changes in the high-temperature region of the glow curve after exposure to densely ionizing radiation (20), has been proposed as a method of evaluating LET (26). However, our study noted no significant relationship between the high temperature region of the glow curve and LET. The annealing procedure, readout parameters, type of TL system, and glow curve analysis method could be factors causing the discrepancy.
For the majority of clinical cases, the TLD-100 would be an effective in vivo dosimetry tool; however, for instances with lower proton energy or for tumors presenting at the surface, such as in breast cancer, the dose uncertainty may increase. The results revealed here are acceptable, although the tolerance of dose uncertainty for each hospital site should be considered. The MCP-100 seems to have the ability to evaluate LET, as it is sensitive to the proton energy spectrum changes. However, one feature of the MCP-100 is its high-dose sensitivity, which means a higher TL response obtained compared to TLD-100 at the same physical dose; thus, photomultiplier tube (PMT) saturation may occur if the dose exceeds a certain dose level, which may be affected by the dose limitation of the TL readout system.
As Figure 3 shows relative efficiency dropped dramatically due to proton energy loss and partial volume effect in the TLD chips with a certain thickness. Low energy proton beams will stop in the crystal and correction factor need to be applied (24). To collect more precise data, thinner TLDs should be used. For the high-energy proton dominated spectrum, the TLD-100 may be an effective dosimetry tool; although, for the low-energy proton dominated spectrum, a higher dosimetry uncertainty should be noted. MCP-100 is highly sensitive to radiation, with a dense ion beam, an under-response is exhibited, resulting a lower dose than expected at the Bragg peak region. The influence of energy dependence should be taken into account when applying the MCP-100 as a proton dosimetry tool; otherwise, the accuracy of the results may be compromised. Thus, the MCP-100 may not be suitable to measure the absolute dose with low-energy proton beams, while the energy dependence must be corrected for. The aforementioned dose difference includes the proton range uncertainty and set-up uncertainty for this experiment, especially at the high-dose gradient region of the Bragg peak.
Conclusions
This study demonstrates the respective proton dosimetry abilities of the TLD-100 and MCP-100. For a high-energy dominated proton beam, the TLD-100 is an effective tool for absolute dose measurement, regardless of a wide-ranging energy spectrum at the measurement point. For a low-energy proton beam, the influence of energy dependence must be taken into account; otherwise, the uncertainty will increase up to 15.18%. MCP-100 may not be suitable for proton dose assessment unless the energy spectrum of the point is realized. Although, in clinical applications including in vivo dose measurement, it is difficult to predict the proton energy spectrum, and subsequently correct for the dose perturbation caused by different proton energies. The uncertainties surrounding dose measurement shown in this study include the influence of energy dependence for both types of TLDs. The improvement of dose measurement accuracy and understanding of the various factors influencing uncertainty are essential. This study further notes the potential of using the MCP-100 for LET measurement.
Acknowledgments
The authors would like to thank James Waddell for the revision of the English language content of this manuscript and all members of the medical physicist team at the Proton and Radiation Therapy Center of Chang Gung Memorial Hospital for their guidance and use of their equipment and facilities.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Chin-Cheng Chen, Dennis Mah, Chang Chang, Joseph Chang and Nancy Lee) for the series “Pencil Beam Scanning Particle Therapy” published in Therapeutic Radiology and Oncology. The article has undergone external peer review.
Data Sharing Statement: Available at https://tro.amegroups.com/article/view/10.21037/tro-22-16/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tro.amegroups.com/article/view/10.21037/tro-22-16/coif). The series “Pencil Beam Scanning Particle Therapy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- DeLaney TF, Kooy HM. Proton and Charged Particle Radiotherapy. Lippincott Williams and Wilkins, 2008.
- Bortfeld T, Paganetti H, Kooy H. MO-A-T-6B-01: Proton Beam Radiotherapy — The State of the Art. Med Phys 2005;32:2048-9. [Crossref]
- Anderson SE, Grams MP, Wan Chan Tseung H, et al. A linear relationship for the LET-dependence of Gafchromic EBT3 film in spot-scanning proton therapy. Phys Med Biol 2019;64:055015. [Crossref] [PubMed]
- Yasui K, Omachi C, Nagata J, et al. Dosimetric response of a glass dosimeter in proton beams: LET-dependence and correction factor. Phys Med 2021;81:147-54. [Crossref] [PubMed]
- Zullo JR, Kudchadker RJ, Zhu XR, et al. LiF TLD-100 as a dosimeter in high energy proton beam therapy--can it yield accurate results? Med Dosim 2010;35:63-6. [Crossref] [PubMed]
- Kry SF, Alvarez P, Cygler JE, et al. AAPM TG 191: Clinical use of luminescent dosimeters: TLDs and OSLDs. Med Phys 2020;47:e19-51. [Crossref] [PubMed]
- Olko P, Currivan L, van Dijk JW, et al. Thermoluminescent detectors applied in individual monitoring of radiation workers in Europe--a review based on the EURADOS questionnaire. Radiat Prot Dosimetry 2006;120:298-302. [Crossref] [PubMed]
- Pradhan AS. Thermoluminescence Dosimetry and its Applications. Radiat Prot Dosimetry 1981;1:153-67.
- Engström PE, Haraldsson P, Landberg T, et al. In vivo dose verification of IMRT treated head and neck cancer patients. Acta Oncol 2005;44:572-8. [Crossref] [PubMed]
- Omanwar SK, Koparkar KA, Virk HS. Recent Advances and Opportunities in TLD Materials: A Review. Defect and Diffusion Forum 2013;347:75-110.
- Kortov V. Materials for thermoluminescent dosimetry: Current status and future trends. Radiation Measurements 2007;42:576-81. [Crossref]
- Tsai HY, Sung CH, Chen HH, et al. Clinical application of ionization density dependence of the glow curve for measuring linear energy transfer in therapeutic proton beams. Radiation Measurements 2019;127:106146. [Crossref]
- Massillon-JL G. Gamboa-deBuen I, Brandan ME. TL response of LiF : Mg,Ti exposed to intermediate energy 1H, 3He, 12C, 16O and 20Ne ions. Journal of Physics D: Applied Physics 2007;40:2584-93. [Crossref]
- Bilski P, Olko P, Budzanowski M, et al. Optimisation of LiF:Mg,Ti Detectors for Dosimetry in Proton Radiotherapy. Radiat Prot Dosimetry 1999;85:367-72. [Crossref]
- Sądel M, Bilski P, Swakoń J, et al. Comparative investigations of the relative thermoluminescent efficiency of LiF detectors to protons at different proton therapy facilities. Radiation Measurements 2015;82:8-13. [Crossref]
- Sądel M, Bilski P, Swakoń J, et al. Relative thermoluminescent efficiency of LiF detectors for proton radiation: Batch variability and energy dependence. Radiation Measurements 2013;56:205-8. [Crossref]
- Bilski P. On the correctness of the thermoluminescent high-temperature ratio (HTR) method for estimating ionization density effects in mixed radiation fields. Radiation Measurements 2010;45:42-50. [Crossref]
- Datz H, Horowitz YS, Oster L, et al. Characteristics of the high temperature thermoluminescence in LiF:Mg,Ti (TLD-100): The effects of batch history. Radiation Measurements 2010;45:710-2. [Crossref]
- Noll M, Böck E, Schöner W, et al. Correlation of the LET-dependent TL-response of LIF:Mg,Ti TL-dosemeters and gentoxic endpoints after proton irradiation. Appl Radiat Isot 2000;52:1135-8. [Crossref] [PubMed]
- Vana N, Schöner W, Fugger M, et al. Absorbed Dose Measurement and LET Determination with TLDs in Space. Radiat Prot Dosimetry 1996;66:145-52. [Crossref]
- Aso T. Geant4 Medical Applications. 2012. Available online: https://wiki.kek.jp/display/g4med/PTSIM
- Akagi T, Aso T, Faddegon B, et al. The PTSim and TOPAS Projects, Bringing Geant4 to the Particle Therapy Clinic. Progress in Nuclear Science and Technology 2011;2:912-7. [Crossref]
- Gieszczyk W, Bilski P, Olko P, et al. Evaluation of the relative thermoluminescence efficiency of LiF:Mg,Ti and LiF:Mg,Cu,P TL detectors to low-energy heavy ions. Radiation Measurements 2013;51-52:7-12. [Crossref]
- Sądel M, Bilski P, Swakoń J, et al. Evaluation of the relative TL efficiency of the thermoluminescent detectors to heavy charged particles. Radiat Prot Dosimetry 2016;168:27-32. [Crossref] [PubMed]
- Trioloa A, Brai M, Bartolotta A, et al. Glow curve analysis of TLD-100H irradiated with radiation of different LET: Comparison between two theoretical method. Nuclear Instruments and Methods in Physics Research A 2006;560:413-7. [Crossref]
- Bilski P, Gieszczyk W, Obryk B, et al. Comparison of commercial thermoluminescent readers regarding high-dose high-temperature measurements. Radiation Measurements 2014;65:8-13. [Crossref]
Cite this article as: Chen YS, Wu SW, Huang HC, Chen HH. Absolute dose measurement and energy dependence of LiF dosimeters in proton therapy beam dosimetry. Ther Radiol Oncol 2022;6:14.


