
Case report of a rare primary intracranial malignant melanoma, reviewing the literature and guidance for management
Introduction
Primary intracranial malignant melanoma (PIMM), is a rare intracranial malignancy arising from melanocytes lining leptomeningeal tissue. It accounts for approximately 1% of all melanomas and 0.07% of all brain malignancies (1). It can be classified into 2 types—diffuse meningeal melanomatosis or solitary (discrete) solid tumours depending on imaging pattern (2). PIMMs rarely metastasize beyond the central nervous system (CNS) and are histologically similar to melanomas of other sites. They are often diagnosed after excluding the presence of extracranial lesions from careful physical examinations and positron emission tomography-computed tomography (PET-CT) imaging. Specific molecular mutations in GNAQ and GNA11 genes may also be helpful to distinguish PIMMs from metastatic melanoma (3,4). Given the rarity of this disease, there are no standard management guidelines and most of the evidence for treatment comes from shared experiences of case reports or are inferred from the treatment of metastatic melanoma. We present a recent case experience in accordance with the CARE reporting checklist (available at https://tro.amegroups.com/article/view/10.21037/tro-21-32/rc) and referenced the evidence to guide our management decisions.
Case presentation
The patient is a 41-year-old female who presented to the ophthalmologist with complaints of blurring of vision in the right eye for a week. On further questioning, she also revealed that she has a history of right-sided headaches, which has worsened in the past month. She also had a history of right hyperopia and amblyopia for which she was treated with laser-assisted in situ keratomileusis (LASIK) at another institution 6 years ago.
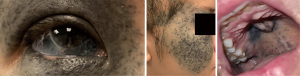
On examination, she has Fitzpatrick skin type IV and a congenital nevus of Ota causing dark pigmentation over the right upper and lower eyelids, sclera and hard palate (Figure 1). Her visual acuity was 6/60 in the right eye (compared to 6/6 on the left). There was right-sided Horner’s syndrome (miosis and partial ptosis), proptosis and grade 3 relative afferent pupillary defect (RAPD). The inferior visual field of the right eye was blurred. There was also a right disc melanocytoma. Extraocular movements were preserved and the remaining cranial nerve examinations were unremarkable.
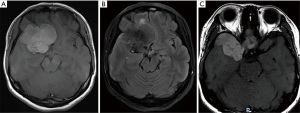
A magnetic resonance imaging (MRI) scan of the brain and orbits showed a large right sphenoid wing extra-axial tumour (measuring 5.8×4.3×4.8 cm) abutting both pre-chiasmatic optic nerves and partially encasing the optic chiasm (Figure 2A-2C). There was an associated mass effect with 1.2 cm leftward midline shift, mild right uncal herniation and mild right frontal lobe oedema. There was no leptomeningeal enhancement pre-operatively nor any tumour apoplexy. The initial diagnosis was that of a large sphenoid wing meningioma.
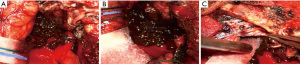
After consultation with her neurosurgeons, the patient decided on a craniotomy and resection. Interestingly, the surgeons encountered a soft black tumour upon opening of the dura (Figure 3A-3C). The surrounding dura and frontal bone were also noted to have black pigmentation. Piecemeal resection was performed and the tumour was cleared from bilateral optic nerves and intracranial vessels. Gross total resection was achieved of the tumour bulk. However, the dura overlying the cavernous sinus and lesser wing of sphenoid were covered by extensive black pigmentation and could not be safely removed. This area of apparent dural origin was therefore extensively coagulated. A frozen section of the specimen was also sent during the surgery which showed a cellular lesion with abundant pigmentation.
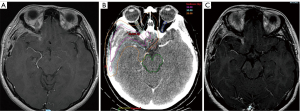
MRI postoperatively (Figure 4A) confirmed the extent of tumour resection with a minimal small focus of dural enhancement along the right planum sphenoidale and leptomeningeal enhancement in the surgical bed, indeterminate for post-operative changes or residual disease.
Histological findings were consistent with that of a meningeal melanoma. The sections showed fragments of pigmented tumour cells in nests, lobules and sheets, sometimes with a fragmented pseudopapillary appearance. Although there was no marked pleomorphism, there was some degree of nuclear irregularity and conspicuous nucleoli. The mitotic activity was around 2–4 per 10 high-power-field at maximum. There was no definite necrosis and no definite brain parenchymal invasion seen. Immunohistochemistry showed the tumour stained positive for S100, SOX10, HMB45 (melanoma antibody) and melan-A. Proliferation index with Ki67 was difficult to assess given obscuring by melanin pigment, but estimated to be between 5–10%. Next generation sequencing (NGS) showed the tumour was microsatellite stable (MSS), and had the following mutations: GNAQ p.Q209P, CDKN2A, CDKN2B deep deletion, SF3B1 p.R625H. The tumour mutation burden was 2.1 mut/MB.
A detailed clinical examination of the patient’s entire skin and mucous membranes by a dermatologist did not find any evidence of cutaneous melanomas. PET-CT scan did not show any suspicious lesions either at the surgical bed or systemically, thereby reaffirming the diagnosis of a PIMM.
Post-operatively, the patient reported marked improvement in the right vision. Repeat examination by the ophthalmologist showed visual acuity 6/7.5 on the right and 6/6 on the left and a near normal visual field. However, there was still a right Horner’s syndrome and grade 2 RAPD.
A multi-disciplinary discussion felt that given the rarity of the case and as the genomic profile (indicated by the presence of a GNAQ mutation) of the tumour appeared similar to uveal melanoma, there might be limited sensitivity and benefit of adjuvant systemic treatment. As there was still possible unresectable residual disease, further local therapy with adjuvant radiotherapy was thus recommended. This was discussed with the patient who understood the rarity of her condition and agreed to share her details for case reporting.
To reduce local recurrence, the patient underwent hypofractionated stereotactic radiotherapy of 30 Gy in 6-daily fractions to the surgical cavity with a simultaneous integrated boost dose of 36 Gy to residual disease and higher risk areas at 2-month post-operation (Figure 4B), taking care to reduce the dose at the optic nerve and chiasm. The patient tolerated the procedure well and had no acute toxicities.
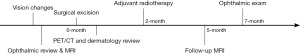
The patient remains on follow-up with us with 3-monthly MRI brain imaging as well as ophthalmic reviews. Her 5-month follow-up scan (Figure 4C) showed reduction of the enhancing lesion without any evidence of local recurrence. Her 7-month ophthalmic examination showed continued improvements in vision with a normal visual field and improved RAPD. The patient remains functional and well. She will however still need regular close follow-up and surveillance scans as there is a high risk of relapse in this disease course. The timeline has been summarized in Figure 5.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
As PIMM is very uncommon, it is important to rule out metastasis from a melanoma of a different site with detailed eye, skin and mucous membrane examinations. There are a few hypotheses regarding the histogenesis of melanocytes within the CNS. The mesodermal theory suggests that the mesodermally-derived pigment cells travel to the CNS via hematogenous spread. The ectodermal theory believes that CNS melanocytic lesions arise from aberrant embryonic ectodermal cells. The neurogenic theory holds that the pigment cells originate from the neural crest, developing into mesodermal and neural elements and giving rise to tumours (5).
Our patient had a congenital nevus of Ota, also known as oculodermal melanocytosis. This is an uncommon benign condition in which proliferation of dermal melanocytes results in a bluish hyperpigmentation in the V1 and V2 trigeminal nerve distribution. It has been described in association with glaucoma, benign melanocytoma, as well as melanomas of the skin, orbits and CNS. The risk level is undefined although it is suspected to be elevated in patients with scleral pigmentation (6) or mutations in the GNAQ and BAP1 gene (7). It is also important to note that lesions may arise contralateral to the nevus of Ota. Due to the risk of malignant transformation, guidelines thus recommend these patients to have an annual screening by a dermatologist and ophthalmologist to pick up early lesions (4,8).
A systematic review in 2018 by Arai et al. (1) showed that PIMM can arise from both supratentorial and infratentorial locations, with frontal lobe and pineal gland locations being the most common. The frontal lobe location was unsurprising due to its larger anatomical size compared to other lobes but the pineal gland location was felt to have been a possible result of publication bias. Other findings from that review include a peak incidence in the fourth and fifth decades of life and no significant gender predisposition. Intracranial hypertension was a common presentation (29.6–60%). Focal neurological deficits and tumour hemorrhage or apoplexy was also noted to be commonly present (1-3).
We are not aware of any international management guidelines specific for PIMM, and its rarity precludes the investigation of specific treatment options in a prospective manner. Given the general treatment-resistant nature of melanomas, surgery should be the mainstay of treatment. This may be followed by adjuvant radiotherapy and/or chemotherapy. The prognosis is generally poor but tends to be better than that of patients with metastatic melanoma. The reported median survival ranges between 11 to 31 months (8,9).
The goal of surgery should be for maximal safe resection. In a literature review, Arai et al. (1) reports a significant survival advantage with gross total resection compared to other types of resections (>22 vs. 12 months, P=0.026). This is congruent with other reported series (2,3,10,11). Other reported prognostic factors include leptomeningeal spread, tumour location, raised intracranial pressure, performance status, tumour bleed and mitotic index (1-3,10,11). However, due to the heterogeneous nature of retrospective series, these factors may not be entirely independent.
The role of definitive and adjuvant radiotherapy is uncertain. Most authors advocate its use to provide a more durable control, especially in the setting of residual disease (3,11,12). In a large heterogeneous series, Li et al. (2) noted a significant survival benefit in patients who were given radiotherapy (HR =0.577; P=0.024). Various treatment techniques and regimens in PIMM have been tried but not compared. In metastatic brain lesions, stereotactic radiotherapy, which delivers a conformal high dose of radiation per fraction to targeted lesions, has been shown to have better neurocognitive preservation and potentially even better lesion control when added to whole brain radiotherapy (13,14). Melanomas as a group are historically considered to be highly radioresistant tumours and thus may benefit from higher doses per fraction to improve local control (15,16). For gross and residual disease, a dose of 60–70 Gy or higher may be recommended to overcome the radioresistance (17). For our patient, a stereotactic hypofractionated regimen of 6×6 Gy (α/β =2.5, EQD2 68 Gy) was given to the residual enhancing areas and a wide dural clinical target volume margin was given a dose of 6×5 Gy in view of the high risk of leptomeningeal spread in these tumours. In our patient’s short duration of follow-up, this dose has appeared to provide a benefit of local control with no toxicities reported thus far.
Melanoma is a largely chemoresistant tumour and the blood-brain barrier itself poses an additional challenge for drug penetration into the CNS. The role of chemotherapy in PIMM remains undefined. A review by Li et al. (2) reported a significant improvement in survival in patients who had received adjuvant chemotherapy HR =0.42 (95% CI: 0.24–0.735), however, this benefit was not seen in other reviews (1). In the literature, multiple agents including nimustine (ACNU), high dose of interferon (IFN) or IFN alpha-2b, semustine (methyl-CCNU), intrathecal methotrexate, and vincristine have been reported with variable outcomes (1,2,9,12). The most common agent used in intracranial melanomas has been dacarbazine but there is some suggestion that temozolomide is more tolerable and may provide a more durable control (12). Kinase inhibitors targeting BRAF and MEK or immune-checkpoint inhibitors (ICI) have shown efficacy in melanoma brain metastases (18). However, ICIs have shown only modest benefit in uveal melanoma (uveal melanomas do not harbour BRAF mutations). The efficacy of ICIs or targeted agents in PIMMs described in case reports has also been mixed (8,19,20). Nevertheless, it remains a promising option in PIMM. More recently, tebentafusp, a new class of drug, has shown survival benefit in selected patients with metastatic uveal melanoma and could also be explored in PIMM.
Conclusions
We report an uncommon case of a PIMM arising from a background nevus of Ota. Given the rarity of this condition, there are no standard treatment options. Our patient was treated with subtotal resection followed by adjuvant stereotactic radiotherapy to the tumour bed. At the 5-month MRI there was regression and stabilization of the lesion and the patient remained well on 7-month follow-up. Literature review reports PIMMs to have an overall aggressive behavior with early relapse though there have been reports of a few long-term survivors (2). The available evidence suggests that the management of solitary lesions should entail aggressive surgical resection followed by adjuvant radiotherapy with or without systemic treatments. The use of newer targeted agents or immunotherapy remains exploratory. Management should be guided by a multi-disciplinary team discussion with the patient and close post-treatment imaging follow-up should be performed due to a high risk of relapse. Further reports using longer-term pooled experiences should continue to be accumulated to guide future management.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tro.amegroups.com/article/view/10.21037/tro-21-32/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tro.amegroups.com/article/view/10.21037/tro-21-32/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Arai N, Kagami H, Mine Y, et al. Primary Solitary Intracranial Malignant Melanoma: A Systematic Review of Literature. World Neurosurg 2018;117:386-93. [Crossref] [PubMed]
- Li CB, Song LR, Li D, et al. Primary intracranial malignant melanoma: proposed treatment protocol and overall survival in a single-institution series of 15 cases combined with 100 cases from the literature. J Neurosurg 2019;132:902-13. [Crossref] [PubMed]
- Huang YM, Yeh KY, Chen PY, et al. Primary intracranial malignant melanomas in solitary type: a tertiary center experience. J Clin Neurosci 2022;101:37-46. [Crossref] [PubMed]
- Blundell AR, Moustafa D, Samore WR, et al. Fatal GNAQ-mutated CNS melanoma in an adolescent with nevus of Ota. Pediatr Dermatol 2021;38:497-9. [Crossref] [PubMed]
- PAPPENHEIM E. BHATTACHARJI SK. Primary melanoma of the central nervous system. Clinical-pathological report of a case, with survey and discussion of the literature. Arch Neurol 1962;7:101-13. [Crossref] [PubMed]
- Williams NM, Gurnani P, Labib A, et al. Melanoma in the setting of nevus of Ota: a review for dermatologists. Int J Dermatol 2021;60:523-32. [Crossref] [PubMed]
- Konstantinov NK, Berry TM, Elwood HR, et al. Nevus of Ota associated with a primary uveal melanoma and intracranial melanoma metastasis. Cutis 2018;102:E2-4. [PubMed]
- Tanoue N, Ummah FC, Hanada T, et al. Incidentally Found Primary Cerebral Malignant Melanoma Associated with Ota Nevus—Wide Dissemination after an Initial Phase of Slow Growth. Hiroshima Journal of Medical Sciences 2018;67:21-9.
- Byun J, Park ES, Hong SH, et al. Clinical outcomes of primary intracranial malignant melanoma and metastatic intracranial malignant melanoma. Clin Neurol Neurosurg 2018;164:32-8. [Crossref] [PubMed]
- Rodriguez y Baena R, Gaetani P, Danova M, et al. Primary solitary intracranial melanoma: case report and review of the literature. Surg Neurol 1992;38:26-37. [Crossref] [PubMed]
- Wang J, Guo ZZ, Wang YJ, et al. Microsurgery for the treatment of primary malignant intracranial melanoma: a surgical series and literature review. Eur J Surg Oncol 2014;40:1062-71. [Crossref] [PubMed]
- Balakrishnan R, Porag R, Asif DS, et al. Primary Intracranial Melanoma with Early Leptomeningeal Spread: A Case Report and Treatment Options Available. Case Rep Oncol Med 2015;2015:293802. [Crossref] [PubMed]
- Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, andomized, controlled, phase 3 trial. Lancet Oncol 2017;18:1049-60. [Crossref] [PubMed]
- Sperduto PW, Shanley R, Luo X, et al. Secondary analysis of RTOG 9508, a phase 3 randomized trial of whole-brain radiation therapy versus WBRT plus stereotactic radiosurgery in patients with 1-3 brain metastases; poststratified by the graded prognostic assessment (GPA). Int J Radiat Oncol Biol Phys 2014;90:526-31. [Crossref] [PubMed]
- Youland RS, Blanchard ML, Dronca R, et al. Role of radiotherapy in extracranial metastatic malignant melanoma in the modern era. Clin Transl Radiat Oncol 2017;6:25-30. [Crossref] [PubMed]
- Bentzen SM, Overgaard J, Thames HD, et al. Clinical radiobiology of malignant melanoma. Radiother Oncol 1989;16:169-82. [Crossref] [PubMed]
- Strojan P. Role of radiotherapy in melanoma management. Radiol Oncol 2010;44:1-12. [Crossref] [PubMed]
- Bander ED, Yuan M, Carnevale JA, et al. Melanoma brain metastasis presentation, treatment, and outcomes in the age of targeted and immunotherapies. Cancer 2021;127:2062-73. [Crossref] [PubMed]
- Troya-Castilla M, Rocha-Romero S, Chocrón-González Y, et al. Primary cerebral malignant melanoma in insular region with extracranial metastasis: case report and review literature. World J Surg Oncol 2016;14:235. [Crossref] [PubMed]
- Fujimori K, Sakai K, Higashiyama F, et al. Primary central nervous system malignant melanoma with leptomeningeal melanomatosis: a case report and review of the literature. Neurosurg Rev 2018;41:333-9. [Crossref] [PubMed]
Cite this article as: Chia BSH, Leow YG, Kumar AA, Keong NC, Farid M. Case report of a rare primary intracranial malignant melanoma, reviewing the literature and guidance for management. Ther Radiol Oncol 2022;6:8.


