Prognostic significance of the preoperative hematological parameters in non-metastatic rectal cancer patients undergoing neoadjuvant chemoradiotherapy and radical surgery
Introduction
Approximately 149,500 newly diagnosed cases of colorectal cancer (CRC) are recorded annually in America, with approximately 52,980 of them ending in death (1). In Taiwan, more than 16,000 people were diagnosed with CRC in 2018. Radical surgery is the main curative treatment for CRC. For T3N0, T4N0, T(any)N+, or locally unresectable rectal cancer, neoadjuvant chemoradiotherapy (NACRT) followed by radical resection is the standard treatment (2,3). However, despite combined-modality treatments, it is difficult to predict outcomes for patients after NACRT.
Studies have shown that cancer-associated inflammation is associated with poorer outcomes, and that hematological inflammatory markers correlate with survival in rectal cancer patients undergoing NACRT (4-6). For some malignant solid tumors, such as CRC, several immune-inflammation measures have been reported to predict prognosis, including the neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), monocyte-lymphocyte ratio (MLR), and systemic immune-inflammation index (SII) (7,8). The purpose of this study was to evaluate the prognostic significance of preoperative hematological parameters, including the absolute neutrophil count (ANC), NLR, PLR, MLR, and SII, in overall survival (OS) of rectal cancer patients receiving trimodal therapy. We present the following article in accordance with the STROBE reporting checklist (available at https://tro.amegroups.com/article/view/10.21037/tro-21-35/rc).
Methods
Patients
A single institution’s cancer registry, Changhua Christian Hospital, was reviewed retrospectively. Patients were identified whom had the American Joint Committee on Cancer (AJCC) cancer staging system 7th edition (9) T3N0, T4N0, T(any)N+, or locally unresectable rectal cancer who underwent NACRT followed by radical surgery between January 2010 and December 2018. The observation period was from January 2010 to October 2021. All patients were at least 20 years old, had histologically confirmed primary rectal cancer, and had no distant metastases when diagnosed. The staging workup included abdominal computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography/computed tomography (PET/CT) scans. Patients were excluded from the study if they had a history of another malignancy before the rectal cancer diagnosis or a history of pelvic irradiation. None of the patients included in the study had autoimmune disease. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Our institutional review board approved this study (CCH IRB No. 200812) and individual consent for this retrospective analysis was waived because the research presented no more than minimal risk.
Data collection
The relevant demographic information and clinical and pathologic variables of each patient were extracted from the institutional records. Hematological parameters that included white blood cell (WBC) counts, hemoglobin (Hb) levels, platelet counts, neutrophil percentages, lymphocyte percentages, monocyte percentages, SII (platelet count × neutrophil count/lymphocyte count), NLR, PLR, and MLR, were collected before treatment, during the NACRT, and perioperatively. Perioperatively means before surgery and 4–6 weeks after operation. The OS was defined as the period from the date of the diagnostic biopsy until death or the end of the follow-up period. The follow-up time was defined as the period from the date of operation until death or the end of the follow-up period.
Treatment
Based on the clinical situation and the physician’s experience, patients received 5-fluorouracil-based chemotherapy, including oral UFUR (tegafur 100 mg/uracil 224 mg)/oral capecitabine or FL (fluorouracil + leucovorin)/FOLFOX (folinic acid + fluorouracil + oxaliplatin)/FOLFIRI (irinotecan + fluorouracil + folinic acid)/CapeOx (capecitabine + oxaliplatin), orally or via intravenous infusion.
Concurrent radiotherapy—including three-dimensional conformal radiation therapy, intensity-modulated radiation therapy (IMRT), image-guided intensity-modulated radiation therapy (IG-IMRT), volumetric-modulated arc therapy (VMAT), and image-guided volumetric modulated arc therapy (IG-VMAT)—was delivered to the whole pelvis, with or without intensity-modulated radiotherapy with simultaneous integrated boost (IMRT-SIB) or VMAT with simultaneous integrated boost (VMAT-SIB) to the primary gross tumor. Radiotherapy was administered in a daily fraction of 1.8–2 Gy/day, 5 days a week, for 5–6 weeks. All treatment plans were generated using the Pinnacle3 Treatment Planning System (Philips; Fitchburg, WI, USA) version 9.6, 9.8, or 9.16. The target volume included the primary tumor, perirectal fat tissue, mesorectum, and presacral and pelvic lymph nodes.
Surgical intervention was performed 12 weeks after the completion of radiotherapy. The surgical methods depended on the surgeon’s clinical judgment and included radical proctectomy with or without coloanal anastomosis, low anterior resection, anterior resection, abdominal perineal resection, transanal endoscopic microsurgery, restorative proctectomy with coloanal anastomosis, and Hartmann’s operation.
Statistical analysis
The Cox proportional hazard model and Kaplan-Meier curve analysis were used to assess the OS. Univariable factors that suggested an association with OS were selected as variables in a multivariate Cox proportional hazard model. The receiver operating characteristic (ROC) curve with the Youden index was chosen as the threshold to dichotomize continuous variables. Statistical significance was set at P<0.05. IBM SPSS v.25 software (IBM; Armonk, NY, USA) was used for all data analyses.
Results
The details of the enrollment diagram are shown in Figure 1. Overall, 96 patients were enrolled in the study, including 70 men (72.9%) and 26 women (27.1%). The patient characteristics are summarized in Table 1. The median age of all patients was 58 years (range, 25–83 years). About 98% of all patients had an Eastern Cooperative Oncology Group (ECOG) performance status score of 0–1, and 2% had a performance status score of 2. Of all patients, 89.6% had moderately differentiated histology. The median initial carcinoembryonic antigen (CEA) concentration was 4.7 ng/dL (range, 0.8–949.6 ng/dL). Nineteen patients had clinical T2 stage tumors, 71 patients had clinical T3 stage tumors, and 6 patients had clinical T4 stage tumors.
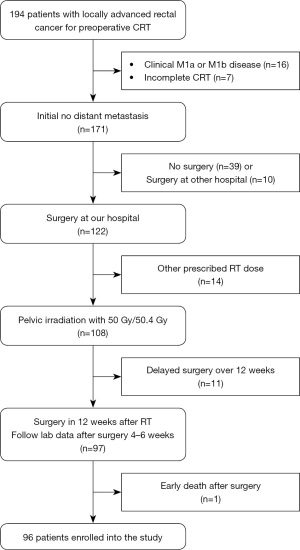
Table 1
| Characteristics | Values (n=96) |
|---|---|
| Age (years), median [range] | 58 [25–83] |
| Follow-up time (months), median [range] | 61.5 [4.0–135.0] |
| Sex, n (%) | |
| Male | 70 (72.9) |
| Female | 26 (27.1) |
| BMI (kg/m2), mean [range] | 23.1 [15.8–33.0] |
| Pretreatment ECOG performance status, n (%) | |
| 0 | 44 (45.8) |
| 1 | 50 (52.1) |
| 2 | 2 (2.1) |
| Differentiation, n (%) | |
| Well differentiated | 3 (3.1) |
| Moderately differentiated | 86 (89.6) |
| Poorly differentiated | 7 (7.3) |
| Distance from anal verge (cm), n (%) | |
| ≤5 | 47 (49.0) |
| 6–15 | 49 (51.0) |
| Clinical T stage, n (%) | |
| T2 | 19 (19.8) |
| T3 | 71 (74.0) |
| T4 | 6 (6.3) |
| Lymph node involvement, n (%) | |
| N0 | 11 (11.5) |
| N1/N2 | 85 (88.5) |
| Clinical stage, n (%) | |
| I | 4 (4.2) |
| II | 7 (7.3) |
| III | 85 (88.5) |
| Interval between preoperative radiotherapy and surgery (days) | |
| Median [range] | 46 [27–75] |
| <42 days, n (%) | 26 (27.1) |
| 42–55 days, n (%) | 53 (55.2) |
| 56–84 days, n (%) | 17 (17.7) |
| Adjuvant chemotherapy, n (%) | 37 (38.5) |
BMI, body mass index; ECOG, the Eastern Cooperative Oncology Group.
All patients received radiotherapy once daily, with either 50 Gy in 25 fractions or 50.4 Gy in 28 fractions, to the whole pelvis and pelvic lymph nodes. The median radiotherapy duration was 38 days (range, 34–55 days). The median period between preoperative radiotherapy and surgery was 46 days (range, 27–75 days).
All patients included in the study received neoadjuvant chemotherapy and the characteristics of the neoadjuvant treatments are summarized in Table 2. Most of the patients tolerated the NACRT course well. Gastrointestinal disease was the most observed complication after neoadjuvant treatment.
Table 2
| Characteristics | Values (n=96) |
|---|---|
| Radiation technique | |
| 3D-CRT, n (%) | 9 (9.4) |
| IMRT, n (%) | 21 (21.9) |
| IG-IMRT, n (%) | 1 (1.0) |
| VMAT, n (%) | 11 (11.5) |
| IG-VMAT, n (%) | 54 (56.3) |
| IMRT-SIB/VMAT-SIB, n (%) | 53 (55.2) |
| CTV-H (Gy), median [range] | 54 [50–56] |
| ≥ Grade 3 toxicity, n (%) | 2 (2.1) |
| Concurrent chemotherapy, n (%) | |
| Oral UFUR/FL | 48 (50.0) |
| Oral capecitabine | 24 (25.0) |
| FOLFOX/FOLFIRI/CapeOX | 24 (25.0) |
3D-CRT, three-dimensional conformal radiotherapy; IMRT, intensity-modulated radiation therapy; IG-IMRT, image-guided IMRT; VMAT, volumetric-modulated arc therapy; IG-VMAT, image-guided VMAT; SIB, simultaneous integrated boost; CTV, clinical target volume; CTV-H, high-risk CTV; UFUR, tegafur 100 mg/uracil 224 mg; FL, fluorouracil + leucovorin; FOLFOX, folinic acid + fluorouracil + oxaliplatin; FOLFIRI, irinotecan + fluorouracil + folinic acid; CapeOX, capecitabine + oxaliplatin.
All patients underwent surgery 12 weeks after finishing the neoadjuvant radiotherapy. The median follow-up time was 61.5 months (range, 4.0–135.0 months). Of all patients, 38.5% (n=37) received adjuvant chemotherapy. The median OS for all patients was 65.0 months (range, 7.0–138.0 months). The 3-year OS rate was 85.4% of all patients.
The univariate parameters included preoperative WBC count, differential counts, Hb level, peripheral platelet count, SII, NLR, PLR and MLR. The differential counts, NLR, PLR and MLR were continuous variable; while the WBC count, Hb level, peripheral platelet count and SII were dichotomized into high and low level. The optimal cut-point values were derived from Youden’s index.
Univariate analysis showed significant associations between poor OS and the preoperative WBC count (>5,200/μL vs. ≤5,200/μL, P=0.004), Hb level (≤11.2 g/dL vs. >11.2 g/dL, P=0.030), peripheral platelet count (>217×103/μL vs. ≤217×103/μL, P=0.002), ANC (P=0.002), NLR (P=0.027), and SII (>656×109/L vs. ≤656×109/L, P=0.008). Preoperative parameters with significant P<0.05 in univariate analysis were included in the multivariate analysis. SII is a composite indicator integrating platelet, neutrophil and lymphocyte counts. To avoid the problems with the presence of multicollinearity, we checked the ROC curve for the SII, preoperative ANC and preoperative NLR. The area under the ROC curve for the SII (0.713) was greater than that for the preoperative ANC (0.674) and preoperative NLR (0.675); therefore, the SII was included in the multivariate analysis.
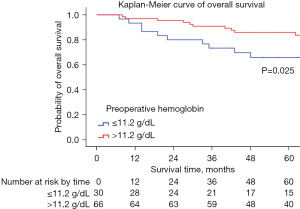
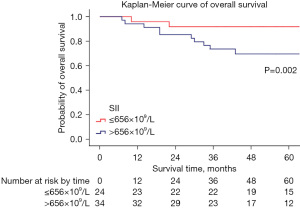
In the multivariate analysis, a high SII [>656×109/L, hazard ratio (HR) 7.293, 95% confidence interval (CI): 1.447–36.765] remained significantly associated with a reduced OS (P=0.016); a high preoperative Hb level (≤11.2 g/dL, HR 0.348, 95% CI: 0.127–0.952) was also associated with a better OS compared with a low level (P=0.040). The characteristics of univariate and multivariate analysis of preoperative hematological parameters for OS are summarized in Table 3. The Kaplan-Meier curves of the OS for the SII and preoperative Hb levels are shown in Figure 2 and Figure 3.
Table 3
| Characteristic | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| P value | HR | 95% CI | P value | HR | 95% CI | ||
| WBC (>5,200/μL, ≤5,200/μL) | 0.004* | 3.365 | 1.485–7.623 | 0.566 | 1.372 | 0.466–4.038 | |
| Preoperative ANC | 0.002* | 1.000 | 1.000–1.001 | – | – | – | |
| Preoperative ALC | 0.969 | 1.000 | 0.999–1.001 | – | – | – | |
| Preoperative AMC | 0.065 | 1.002 | 1.000–1.005 | – | – | – | |
| Hb (≤11.2 g/dL, >11.2 g/dL) | 0.030* | 0.420 | 0.191–0.921 | 0.040* | 0.348 | 0.127–0.952 | |
| Platelet (>217×103/μL, ≤217×103/μL) | 0.002* | 3.477 | 1.573–7.686 | – | – | – | |
| SII (>656×109/L, ≤656×109/L) | 0.008* | 7.427 | 1.683–32.784 | 0.016* | 7.293 | 1.447–36.765 | |
| NLR | 0.027* | 1.156 | 1.017–1.315 | – | – | – | |
| PLR | 0.111 | 1.001 | 1.000–1.003 | – | – | – | |
| MLR | 0.346 | 1.249 | 0.787–1.983 | – | – | – | |
*, P value <0.05. HR, hazard ratio; CI, confidence interval; WBC, white blood cell count; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; AMC, absolute monocyte count; Hb, hemoglobin; SII, systemic immune-inflammation index; NLR, neutrophil-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; MLR, monocyte-lymphocyte ratio.
Discussion
In 1863, Virchow et al. first reported an association between cancer and systemic inflammation (10). The prognostic value of inflammation-related parameters, including the SII, NLR, and PLR, has been demonstrated in several types of solid cancers such as CRC (11-14). In this study, a high SII and a low preoperative Hb level were correlated with poor OS in patients with rectal cancer after NACRT. The SII was a better prognostic factor for survival outcomes than ANC and NLR.
Recently, the SII was suggested to be associated with a poor outcome of cancer, based on counts of neutrophils, lymphocytes, and platelets. Hu et al. (15) demonstrated in two independent cohorts that the preoperative SII is a powerful prognostic predictor of the post-surgical outcomes in patients with hepatocellular carcinoma and might be related to circulating tumor cells. For patients with metastatic CRC who are candidates for first-line chemotherapy plus bevacizumab, the SII is a good prognostic and predictive marker (16). The prognostic value of the SII in patients with CRC after radical surgery was first reported in 2017 (17). Consistent with the results of previous studies, our study found that the SII was an independent predictor of OS and disease-free survival. Based on the area under the curve values obtained from the ROC curves, the SII was a better predictive factor of long-term survival than the preoperative ANC and preoperative NLR; this might be because the SII reflects the states of the immune response and inflammation more comprehensively.
In addition to WBC and peripheral platelet counts, Hb is a hematological parameter that can be checked routinely during treatment. Hypoxia is associated with increased resistance to tumor cell death during radiotherapy and worsens the outcome. Several factors that are represented by the Hb level affect tumor oxygenation, including the adequacy of the blood supply, microcirculation, and the oxygen-carrying capacity of the blood (18). The effect of Hb levels on the response to treatment, measured before and during concurrent chemoradiotherapy (CRT), has been well addressed in cervical cancer (19). Patients with pretreatment Hb levels less than 12 g/dL have potentially worse outcomes for solid tumors (20-22). Furthermore, low Hb levels before or during radiotherapy are associated with a reduced rate of pathological complete regression (pCR) after NACRT for rectal cancer (23). Patients with pCR have a better prognosis (24-26), and the treatment strategy for these patients may differ from that for patients without pCR. Our findings are consistent with those of previous studies that showed that a high SII and low Hb levels are potential markers for predicting survival of rectal cancer patients treated with surgery after NACRT.
However, there were limitations to this study. First, this was a retrospective study, and some hematologic parameters may have been missed in the setting. Second, the different chemotherapy regimens and surgical methods were chosen by the clinical physicians’ preferences, which might have resulted in selection bias. Therefore, prospective studies with a larger number of patients are needed to confirm our findings.
Conclusions
In summary, a high preoperative WBC count, peripheral platelet count, ANC, NLR, and SII were associated with poor OS in rectal cancer patients receiving preoperative CRT followed by radical surgery. Patients with high preoperative Hb levels might have a better prognosis than those with low preoperative Hb levels in rectal cancer after NACRT. Compared with ANC and NLR, the SII was more powerful for predicting OS in patients with rectal cancer after preoperative CRT.
Acknowledgments
We thank our Department of Statistical Sciences and Epidemiology for their assistance and the clinicians who collaborated in the treatment of the patients in this study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tro.amegroups.com/article/view/10.21037/tro-21-35/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tro.amegroups.com/article/view/10.21037/tro-21-35/coif). JCL serves as an unpaid editorial board member of Therapeutic Radiology and Oncology from May 2020 to April 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Changhua Christian Hospital (CCH IRB No. 200812), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926-33. [Crossref] [PubMed]
- Roh MS, Colangelo LH, O'Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 2009;27:5124-30. [Crossref] [PubMed]
- Dudani S, Marginean H, Tang PA, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictive and prognostic markers in patients with locally advanced rectal cancer treated with neoadjuvant chemoradiation. BMC Cancer 2019;19:664. [Crossref] [PubMed]
- Shen J, Zhu Y, Wu W, et al. Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Locally Advanced Rectal Cancer Treated with Neoadjuvant Chemoradiotherapy. Med Sci Monit 2017;23:315-24. [Crossref] [PubMed]
- Zhang X, Li J, Peng Q, et al. Association of markers of systemic and local inflammation with prognosis of patients with rectal cancer who received neoadjuvant radiotherapy. Cancer Manag Res 2018;11:191-9. [Crossref] [PubMed]
- Zou ZY, Liu HL, Ning N, et al. Clinical significance of pre-operative neutrophil lymphocyte ratio and platelet lymphocyte ratio as prognostic factors for patients with colorectal cancer. Oncol Lett 2016;11:2241-8. [Crossref] [PubMed]
- Diakos CI, Charles KA, McMillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493-503. [Crossref] [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Virchow R. Cellular pathology. As based upon physiological and pathological histology. Lecture XVI--Atheromatous affection of arteries. 1858. Nutr Rev 1989;47:23-5. [Crossref] [PubMed]
- Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. [Crossref] [PubMed]
- Templeton AJ, Ace O, McNamara MG, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2014;23:1204-12. [Crossref] [PubMed]
- Zhong JH, Huang DH, Chen ZY. Prognostic role of systemic immune-inflammation index in solid tumors: a systematic review and meta-analysis. Oncotarget 2017;8:75381-8. [Crossref] [PubMed]
- Yang R, Chang Q, Meng X, et al. Prognostic value of Systemic immune-inflammation index in cancer: A meta-analysis. J Cancer 2018;9:3295-302. [Crossref] [PubMed]
- Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res 2014;20:6212-22. [Crossref] [PubMed]
- Passardi A, Scarpi E, Cavanna L, et al. Inflammatory indexes as predictors of prognosis and bevacizumab efficacy in patients with metastatic colorectal cancer. Oncotarget 2016;7:33210-9. [Crossref] [PubMed]
- Chen JH, Zhai ET, Yuan YJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol 2017;23:6261-72. [Crossref] [PubMed]
- Becker A, Stadler P, Lavey RS, et al. Severe anemia is associated with poor tumor oxygenation in head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys 2000;46:459-66. [Crossref] [PubMed]
- Obermair A, Cheuk R, Horwood K, et al. Impact of hemoglobin levels before and during concurrent chemoradiotherapy on the response of treatment in patients with cervical carcinoma: preliminary results. Cancer 2001;92:903-8. [Crossref] [PubMed]
- Grimm T, Buchner A, Schneevoigt B, et al. Impact of preoperative hemoglobin and CRP levels on cancer-specific survival in patients undergoing radical cystectomy for transitional cell carcinoma of the bladder: results of a single-center study. World J Urol 2016;34:703-8. [Crossref] [PubMed]
- Pergialiotis V, Daskalakis G, Thomakos N, et al. Prechemotherapy Hemoglobin Levels as a Predictive Factor of Ovarian Cancer Survival: A Systematic Review and Meta-Analysis. Am J Clin Oncol 2019;42:725-31. [Crossref] [PubMed]
- Beresford MJ, Burcombe R, Ah-See ML, et al. Pre-treatment haemoglobin levels and the prediction of response to neoadjuvant chemotherapy in breast cancer. Clin Oncol (R Coll Radiol) 2006;18:453-8. [Crossref] [PubMed]
- Bong JW, Lim SB, Ryu H, et al. Effect of anaemia on the response to preoperative chemoradiotherapy for rectal cancer. ANZ J Surg 2021;91:E286-91. [Crossref] [PubMed]
- Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010;11:835-44. [Crossref] [PubMed]
- van de Velde CJ, Boelens PG, Borras JM, et al. EURECCA colorectal: multidisciplinary management: European consensus conference colon & rectum. Eur J Cancer 2014;50:1.e1-1.e34. [Crossref] [PubMed]
- Aktan M, Yavuz BB, Kanyilmaz G, et al. Factors affecting pathological response and survival following neoadjuvant chemoradiotherapy in rectal cancer patients. Indian J Cancer 2021;58:553-60. [PubMed]
Cite this article as: Lin YE, Huang SY, Chang TH, Chou TW, Hung LC, Huang CC, Lin JB, Lin JC. Prognostic significance of the preoperative hematological parameters in non-metastatic rectal cancer patients undergoing neoadjuvant chemoradiotherapy and radical surgery. Ther Radiol Oncol 2022;6:5.