
Cardiac dosage comparison among whole breast irradiation and partial breast irradiation techniques
Introduction
The adjuvant whole breast irradiation (WBI) after breast conservative surgery (BCS) is now the standard care for early-stage breast cancer patients (1). From the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) study, adjuvant WBI for early-stage breast cancer patients receiving BCS would have significantly higher loco-regional control survival and overall survival (2). However, long term follow-up indicated that the incidence of major coronary events increased linearly with the mean dose to the heart by 7.4% per Gray (3). In the recent update results of the Danish part of the above study, the linear increase in the excess odds ratio of major coronary events was 19% per Gray in the tangential photon techniques patients, and for the patients treated with electron techniques, there was no significant association between mean heart dose and the risk of major coronary events (4). In recent years, accelerated partial breast irradiation (APBI) has obtained more and more attention due to early diagnosis of breast cancer and the significant reduction of total treatment time (5). Studies have shown that the most common site of tumor recurrence is the region around the tumor bed after surgery in early breast cancer (6,7), therefore relatively large fraction size irradiation over the lumpectomy cavity in a shorter time should have a considerably good local control rate (8). In conventional WBI, it will take around 5–6 weeks for a complete treatment course, whereas APBI can shorten the treatment course to 4–5 days. It is essentially beneficial for the radiation oncologists and the patients especially in the COVID-19 pandemic nowadays (9).
There are several modalities of APBI and the most common techniques are the interstitial brachytherapy (ISBT) and the partial breast external beam radiotherapy (PB-EBRT) (10-12). Compared to the whole breast external beam radiotherapy (WB-EBRT), the APBI can lower the dose of the normal tissue and the organs at risk (OARs) received (e.g., contralateral breast, lung and heart) (13). Recently, cardiovascular toxicity of radiotherapy has been emphasized, therefore decreasing the heart dose should be taken into account especially in the long-lived and left-sided breast cancer patients (14).
However, there are few studies comparing the dosimetry among different techniques of APBI and WBI (15). Furthermore, fewer studies focus on cardiovascular toxicity and dosimetry and the results still remain equivocal (16). The purpose of this study was to compare the dosimetry of the heart, the left anterior descending artery (LAD) and the lung among the ISBT, the PB-EBRT and WB-EBRT. We present the following article in accordance with the STROBE reporting checklist (available at https://tro.amegroups.com/article/view/10.21037/tro-21-27/rc).
Methods
Ethics
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Kaohsiung Veterans General Hospital Institutional Review Board (VGHKS21-CT7-01) and individual consent for this retrospective analysis was waived.
Patient eligibility
We included patients with early stage left breast cancer receiving ISBT from January, 2015 to June, 2021. All patients were treated with multi-catheter interstitial brachytherapy (MIBT) with APBI protocol by high-dose-rate (HDR) Ir-192 stepping source. The selection criteria for ISBT were based on the American Society for Radiation Oncology (ASTRO) APBI guideline consensus statement (11,12). According to these criteria, this study included patients ≥45-year-old, tumor greatest diameter ≤2 cm (pT1 disease), surgical margins ≥2 mm, negative of lymphovascular invasion, positive of estrogen receptor (ER) and negative of nodal metastasis or distant metastasis.
Clinical target volume (CTV) definition and contouring
The computed tomography (CT) scans with 1.25 or 2.5 mm slice thickness were acquired for each patient from lower neck to upper abdomen including bilateral breasts and lungs after the implantation of catheters. The lumpectomy cavity was first delineated, then a margin was added for clinical suspicious tumor spread and pathological tumor-free margin in all three dimensions. In the ISBT plan, the planning target volume (PTV) was considered as CTV because the position relationship between the catheters and the lumpectomy cavity was fixed and there was no setup error in this treatment modality. A digital phantom PB-EBRT plan and a digital phantom WB-EBRT plan was created for each patient based on the same CT images. In the PB-EBRT plan, the PTV was defined as a 10 mm margin applied to CTV (11). In the WB-EBRT plan, the whole breast was contoured as CTV and a margin of 5 mm was added as PTV (17). OARs included ipsilateral and contralateral breasts, lungs, skin, ribs, heart and LAD. The delineation of the cardiovascular system was based on the 2020 Danish Multidisciplinary Cancer Groups (DMCG) National guidelines of delineation of whole heart and substructures in thoracic radiation therapy (18). NSABP B-39/RTOG 04-13 guidelines were used for target and normal tissue constraints (19).
ISBT plan
Total dose of 26–28 Gy in 4 fractions was prescribed. If feasible, two fractions a day were performed for all patients. Oncentra Brachy v4.5.3 (Nucletron, Veenendaal, The Netherlands) planning system was used for contouring, catheters reconstruction and treatment plan. Dosage optimization was done by the planning system first then confirmation by manual adjustment of the radiation oncologists to reduce the hot spot and the non-uniformity of the dose prescription. Treatment plans were accepted if requirements for target and normal tissue were met as at least 90% of PTV coverage under prescribed dose; dose-nonuniformity ratio (DNR) = V150/V100 ≤70%.
Digital phantom PB-EBRT plan
For PB-EBRT, we used the same CT images from ISBT CT simulation. All contours on Oncentra Brachy were transferred to Pinnacle3 v14.0.0 (PHILIPS, Fitchburg, WI, USA). The digital phantom PB-EBRT plan was created for each patient. The CTV and OARs were the same as in the ISBT plan, whereas the PTV in the digital phantom PB-EBRT plan was defined as a 10 mm margin applied to CTV in all directions in consideration of daily setup errors and organ motions, and 4 mm from body surface was excluded from the PTV. The prescribed dose of 38.5 Gy in 10 fractions was set. Volumetric modulated arc therapy (VMAT) with two partial arcs with optimal gantry and collimator rotations were employed to achieve minimal ipsilateral lung and heart irradiation. Each plan was accepted if all the following constraints for coverage, homogeneity and OARs were fulfilled: PTV coverage (D100) ≥95%, D105 ≤5%, ipsilateral lung V30 <15%, contralateral lung V5 <15% and heart V5 <40% (19).
Digital phantom WB-EBRT plan
For WB-EBRT, we used the same CT images from ISBT CT simulation. All contours on Oncentra Brachy were transferred to Pinnacle3 and the whole breast was contoured on Pinnacle3. The digital phantom WB-EBRT plan was created for each patient. The OARs were the same as in the ISBT plan, whereas the CTV was defined as whole breast and the PTV was defined as a 5 mm margin applied to CTV in all directions in consideration of daily setup errors and organ motions, and 4 mm from body surface was excluded from the PTV. The prescribed dose in the digital phantom WB-EBRT plan was given as 50 Gy in 25 fractions. VMAT with two partial arcs with optimal gantry and collimator rotations were employed to achieve minimal ipsilateral lung and heart irradiation. Each plan was accepted if all the following constraints for coverage, homogeneity and OARs were fulfilled: PTV coverage (D100) ≥90%, D105 ≤5%, ipsilateral lung V20 <30% and mean heart dose <10 Gy (20).
Dosimetric assessment and statistical analysis
Dose-volume histograms (DVH) were acquired for analyzing the heart dose for each patient in the ISBT plan, the digital phantom PB-EBRT plan and the digital phantom WB-EBRT plan. Parameters of the maximum dose (Dmax), mean dose (Dmean), dose to certain absolute volumes DVolume/cc (D2 cc, D10 cc and D25 cc) and volumes receiving 5, 10 and 20 Gy (V5 Gy, V10 Gy and V20 Gy) were calculated. As for LAD, which is generally regarded as a serial organ, dose-volume parameters were not applicable, thus the Dmax and Dmean were used for parameter analysis. The parameters of lung were also collected, including Dmax, Dmean, D2 cc, D10 cc, D25 cc, V5 Gy, V10 Gy and V20 Gy.
The dose of the ISBT plan may not be radiobiological comparable to the digital phantom PB-EBRT plan and the digital phantom WB-EBRT plan due to different fraction sizes. Therefore, the dose parameters were calculated to biological equivalents using the linear quadratic equation, and an alpha/beta of 3 Gy was used for late effects to the heart and lung (21).
The mean and standard deviation (SD) for each dosimetric parameter was calculated. The significance of the difference among the above three treatment plans was assessed using ANOVA. The null hypothesis was assumed that there were no significant statistical differences among three groups of treatment plans. If there were significant differences noted among three groups of treatment plans, post-hoc analysis was further applied for comparison between each two groups. All statistical analyses were performed using SPSS Statistics v25. We considered P<0.05 to be statistically significant.
Results
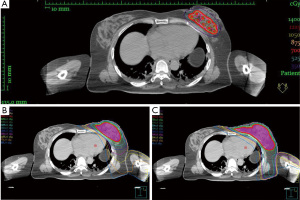
In our institution, 25 breast cancer patients received ISBT treatment, and 12 patients (48%) had left-sided lesions. The mean age of the 12 left-sided breast cancer patients was 57.8±8.3 (mean ± SD) years old and all patients included were female. According to the 8th edition of American Joint Committee on Cancer (AJCC) cancer staging system, 1 patient was ductal carcinoma in situ (DCIS) disease and 11 patients were stage IA [pT1N(sn)0M0] disease (22). Among these 12 patients, 1 (8.3%) was located at upper inner quadrant, 4 (33.3%) were at upper outer quadrant, 3 (25%) were at lower inner quadrant and 4 (33.3%) were at lower outer quadrant. The mean number of catheters inserted was 12.75 (range, 9–20), and the mean plane of catheters was 2.67 (range, 2–4). Eleven patients received a total 28 Gy in 4 fractions, and one patient received 26 Gy in 4 fractions. For the digital phantom PB-EBRT plan, all 12 patients received 38.5 Gy in 10 fractions. For the digital phantom WB-EBRT plan, all 12 patients received 50 Gy in 25 fractions. The characteristics of the patients are summarized in Table 1. The axial view base on CT simulation of the isodose distribution in the same patient of ISBT, PB-EBRT and EB-EBRT were presented in Figure 1.
Table 1
| Variables | Number (%) | Mean ± SD |
|---|---|---|
| Age | – | 57.8±8.3 |
| Gender | ||
| Female | 12 (100.0) | – |
| Male | 0 | – |
| AJCC 8th stage group | ||
| DCIS | 1 (8.3) | – |
| IA (pT1N(sn)0M0) | 11 (91.7) | – |
| Quadrant | ||
| Upper inner | 1 (8.3) | – |
| Upper outer | 4 (33.3) | – |
| Lower inner | 3 (25.0) | – |
| Lower outer | 4 (33.3) | – |
| ISBT doses | ||
| 28 Gy/4 fractions | 11 (91.7) | – |
| 26 Gy/4 fractions | 1 (8.3) | – |
| Digital phantom PB-EBRT doses | ||
| 38.5 Gy/10 fractions | 12 (100.0) | – |
| Digital phantom WB-EBRT doses | ||
| 50 Gy/25 fractions | 12 (100.0) | – |
All the data are original from the study approved by Kaohsiung Veterans General Hospital Institutional Review Board (VGHKS21-CT7-01). AJCC, American Joint Committee on Cancer; DCIS, ductal carcinoma in situ; ISBT, interstitial brachytherapy; PB-EBRT, partial breast external beam radiotherapy; WB-EBRT, whole breast external beam radiotherapy; SD, standard deviation.
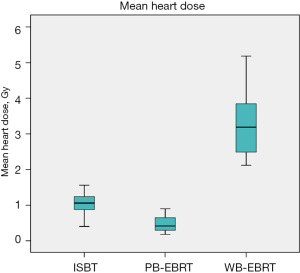
After converting to equivalent dose in 2 Gy fractions (EQD2), the dosimetry of heart for the ISBT plan, digital phantom PB-EBRT plan and digital phantom WB-EBRT plan were presented in Table 2. The mean heart dose in the ISBT plan, the digital phantom PB-EBRT plan and the digital phantom WB-EBRT plan was 1.05, 0.47, 3.24 Gy respectively (P<0.05). A box-plot of the mean heart dose in different types of treatment modalities was presented in Figure 2. Both APBI techniques were significantly lower than the WBI technique (P<0.05), and there was no significant difference between two techniques of APBI (P=0.64). There was also a significant difference in Dmax, D2 cc, D10 cc, and D25 cc among three techniques of radiotherapy (Table 2). Compared to the digital phantom WB-EBRT plan, the dosimetry in both APBI plans was all significantly lower in post-hoc analysis, whereas no significant difference was noted between two groups of APBI (Table 2). For the volume irradiated by the specific dose (5, 10 and 20 Gy), there was also a significant difference in V5 Gy, V10 Gy and V20 Gy among three methods of irradiation, and the parameters in the digital phantom WB-EBRT plan were all significantly higher than the two methods of APBI. There was no significant difference between two methods of APBI (Table 2).
Table 2
| Dosimetric parameter | ISBT [1] (Gy, mean ± SD) | Digital phantom PB-EBRT [2] (Gy, mean ± SD) |
Digital phantom WB-EBRT [3] (Gy, mean ± SD) | P | P (post-hoc) | ||
|---|---|---|---|---|---|---|---|
| 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | |||||
| Dmean | 1.05±0.30 | 0.47±0.23 | 3.24±0.93 | <0.001 | 0.064 | <0.001 | <0.001 |
| Dmax | 9.38±4.14 | 20.46±21.67 | 47.47±5.87 | <0.001 | 0.136 | <0.001 | <0.001 |
| D2 cc | 6.74±2.60 | 10.97±13.19 | 33.15±8.29 | <0.001 | 0.530 | <0.001 | <0.001 |
| D10 cc | 4.85±1.79 | 4.21±4.29 | 18.99±9.25 | <0.001 | 0.967 | <0.001 | <0.001 |
| D25 cc | 3.62±1.24 | 1.64±1.20 | 10.99±7.46 | <0.001 | 0.552 | 0.001 | <0.001 |
| V5 Gy | 1.85±1.71 (%) | 1.08±1.40 (%) | 9.44±4.89 (%) | <0.001 | 0.833 | <0.001 | <0.001 |
| V10 Gy | 0.08±0.11 (%) | 0.57±0.79 (%) | 4.30±2.98 (%) | <0.001 | 0.800 | <0.001 | <0.001 |
| V20 Gy | 0 (%) | 0.21±0.37 (%) | 1.94±1.69 (%) | <0.001 | 0.876 | 0.001 | <0.001 |
All parameters were converted to EQD2. All the data are original from the study approved by Kaohsiung Veterans General Hospital Institutional Review Board (VGHKS21-CT7-01). ISBT, interstitial brachytherapy; PB-EBRT, partial breast external beam radiotherapy; WB-EBRT, whole breast external beam radiotherapy; EQD2, equivalent dose in 2 Gy fractions; SD, standard deviation.
As for the dose of LAD, there was a significant difference in the mean dose among the ISBT plan, the digital phantom PB-EBRT plan and the digital phantom WBEBT plan, and the post-hoc analysis showed that both APBI plans were significantly lower than the digital phantom WB-EBRT plan, meanwhile the ISBT plan was significantly higher than the digital phantom PB-EBRT plan (Table 3). The mean LAD dose in the ISBT plan, the digital phantom PB-EBRT plan and the digital phantom WB-EBRT plan was 1.68, 0.49, 3.34 Gy respectively. Although there was a significant difference in Dmax among three techniques of irradiation, there was no significant difference between the ISBT plan and the digital phantom PB-EBRT plan with P=0.703. The value of Dmax in the ISBT plan, digital phantom PB-EBRT plan and digital phantom WB-EBRT plan was 3.08, 1.80 and 12.13 Gy, respectively (Table 3).
Table 3
| Dosimetric parameter | ISBT [1] (Gy, mean ± SD) | Digital phantom PB-EBRT [2] (Gy, mean ± SD) | Digital phantom WB-EBRT [3] (Gy, mean ± SD) | P | P (post-hoc) | ||
|---|---|---|---|---|---|---|---|
| 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | |||||
| Dmean | 1.68±1.00 | 0.49±0.30 | 3.34±0.68 | <0.001 | 0.001 | <0.001 | <0.001 |
| Dmax | 3.08±2.01 | 1.80±2.34 | 12.13±5.63 | <0.001 | 0.703 | <0.001 | <0.001 |
All parameters were converted to EQD2. All the data are original from the study approved by Kaohsiung Veterans General Hospital Institutional Review Board (VGHKS21-CT7-01). ISBT, interstitial brachytherapy; PB-EBRT, partial breast external beam radiotherapy; WB-EBRT, whole breast external beam radiotherapy; EQD2, equivalent dose in 2 Gy fractions; LAD, left anterior descending artery; SD, standard deviation.
Comparing the lung dosimetry among the ISBT plan, the digital phantom PB-EBRT plan and the digital phantom WB-EBRT plan, all parameters were significantly different among three techniques. In post-hoc analysis, both the ISBT plan and the digital phantom PB-EBRT plan were all significantly lower than the digital phantom WB-EBRT plan (Table 4). There was no significant difference in the Dmean, D10 cc, D25 cc between the ISBT plan and the digital phantom PB-EBRT plan (Table 4). However, the Dmax and D2 cc in the ISBT plan were significantly lower than those in the digital phantom PB-EBRT plan (Table 4). For the volume irradiated by the specific dose, there was significant difference in V5 Gy, V10 Gy and V20 Gy among three techniques, and both APBI groups were all lower than the digital phantom WB-EBRT group, but no significant difference was noted between the ISBT plan and the digital phantom PB-EBRT plan (Table 4).
Table 4
| Dosimetric parameter | ISBT [1] (Gy, mean ± SD) | Digital phantom PB-EBRT [2] (Gy, mean ± SD) | Digital phantom WB-EBRT [3] (Gy, mean ± SD) | P | P (post-hoc) | ||
|---|---|---|---|---|---|---|---|
| 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | |||||
| Dmean | 1.06±0.33 | 1.11±0.51 | 5.76±1.72 | <0.001 | 0.993 | <0.001 | <0.001 |
| Dmax | 14.86±7.06 | 36.87±13.84 | 50.02±2.32 | <0.001 | <0.001 | <0.001 | 0.005 |
| D2 cc | 10.56±5.31 | 23.02±13.06 | 43.34±5.29 | <0.001 | 0.005 | <0.001 | <0.001 |
| D10 cc | 7.37±3.90 | 14.84±11.85 | 36.30±8.79 | <0.001 | 0.132 | <0.001 | <0.001 |
| D25 cc | 5.29±2.87 | 9.49±9.06 | 29.10±11.20 | <0.001 | 0.488 | <0.001 | <0.001 |
| V5 Gy | 3.56±2.81 (%) | 5.83±3.52 (%) | 25.54±9.22 (%) | <0.001 | 0.647 | <0.001 | <0.001 |
| V10 Gy | 0.63±0.85 (%) | 2.59±2.27 (%) | 14.80±6.92 (%) | <0.001 | 0.532 | <0.001 | <0.001 |
| V20 Gy | 0.03±0.09 (%) | 0.77±1.29 (%) | 7.85±4.22 (%) | <0.001 | 0.779 | <0.001 | <0.001 |
All parameters were converted to EQD2. All the data are original from the study approved by Kaohsiung Veterans General Hospital Institutional Review Board (VGHKS21-CT7-01). ISBT, interstitial brachytherapy; PB-EBRT, partial breast external beam radiotherapy; WB-EBRT, whole breast external beam radiotherapy; EQD2, equivalent dose in 2 Gy fractions; SD, standard deviation.
Discussion
In conventional WBI, the standard prescribed dose was suggested 45–50.4 Gy in 25–28 fractions to the whole breast. For left-sided breast cancer patients under this prescription, the mean heart dose could range from 1.29–1.94 to 8.5–8.9 Gy (23,24). The high variability may due to different consistency of manual contouring of the heart structure, the experience and the technique of the treatment and even the anatomy difference in every patient. Several studies had indicated that the risk of myocardial infarction has a linear dose response to the mean heart dose (3,4,23-28). Patients receiving a mean dose of 20 Gy to the heart had a 3.4-fold higher myocardial infarction rate than unirradiated patients, and the cumulative risk of myocardial infarction increased over time from the year when breast cancer was diagnosed (24). Meanwhile, there was also a trend that the excess rate ratio of myocardial infarction was higher for younger women (20,24). This is essentially important because of the early detection of breast cancer and the prolonged survival after surgery and radiotherapy. Reducing the mean heart dose is expected to contribute to lower the cardiovascular events of long-term breast cancer survivors.
APBI has been proven that the long-term ipsilateral breast-tumor recurrence (IBTR) rate was non-inferior to that of WBI in selected patients based on phase 3 randomized trials (8,29). However, long-term follow up of the heart and lung toxicity has not been interpreted yet. Before regular application of APBI treatment, it will be helpful to understand the differences of heart and LAD dosimetry between WBI and APBI. In our study, the mean heart dose was 3.24, 1.05 and 0.47 Gy in the WB-EBRT plan, the ISBT plan and the PB-EBRT plan, respectively. The mean heart doses in both APBI techniques were all significantly lower than that in the WBI technique (P<0.05, Table 2). The mean heart doses in both APBI plans were all lower than the DEGRO recommendation, which suggested the mean heart dose <2.5 Gy (30). For dosimetry comparison, we used the same CT images in the ISBT plan and both digital phantom plans, therefore the dosimetry of the heart in the digital phantom PB-EBRT plan and the digital phantom WB-EBRT plan could be underestimated, because the breast tissue was pulled away from its normal position due to the implantation of the catheters. The distance between the actual tumor bed and the heart was farther compared to preoperative CT images. It was our understanding that the external beam technique could shape the lower isodose lines more apart from the heart due to higher conformal capability. On the other hand, brachytherapy with dose delivery via multi-catheter had less capacity for heart sparing in order not to compromise PTV coverage.
Besides, there were few studies mentioning the dose distribution impact on the LAD. Stenosis of the coronary artery may lead to consequential coronary artery disease (CAD) and the LAD is one of the most important arteries. Nilsson et al. demonstrated that the distribution and extent of coronary stenosis in the heart was correlated to the radiation dose delivered and its location in the heart (31). In our study, the Dmax of the LAD was 3.08 and 1.80 Gy in the ISBT plan and the digital phantom PB-EBRT plan respectively with no significant difference and the Dmax was 12.13 Gy in the digital phantom WB-EBRT plan. In a left-sided breast cancer patient receiving irradiation, the LAD and the left ventricle usually lie in the high dose region due to the anatomic location of the heart. The LAD is situated at the surface of the left heart, which is nearest the chest wall, therefore the LAD would receive higher dose despite slope dose gradient.
In a study by Chan et al., the authors performed the dosimetry comparison between multi-catheter APBI and WB-EBRT (15). The maximum dose of LAD was 6.0 and 45.9 Gy respectively (P<0.05). The dose in the ISBT plan was significantly lower than the WB-EBRT plan. To our knowledge, there was no study comparing the radiation dose to the LAD in different APBI methods. In our study, we found that no matter which technique of APBI was used, both could greatly reduce the radiation dose to the LAD, suggesting possibly decreasing the risks of CAD.
In WBI, all parameters of lung dosimetry were significantly higher than both APBI techniques, and the Dmax, Dmean and V20 Gy could reach 50.02, 5.76 and 7.85% respectively. We noticed that in the lung dosimetry compared between the ISBT plan and the digital phantom PB-EBRT plan, the dose in the ISBT plan was significantly lower than in the digital phantom PB-EBRT plan in the doses to small volumes (Dmax and D2 cc), and no significant difference when the volumes increased. According to the characteristic of partial breast irradiation, the ipsilateral lung volume receiving radiation exposure was confined in a certain region. As a consequence, when the volume increased, the ratio of the dose taken into account would then decrease. In addition, in order to spare the heart receiving additional exposure dose and by the limitation of the physics of radiation, the lung would receive an additional low dose in compensation in the digital phantom PB-EBRT plan.
One of the remarkable studies performed by Major et al. conducted a dosimetric comparison using MIBT and intensity modulated radiotherapy (IMRT) for APBI with focus on dosimetry to normal tissues and OARs (32). This was the first study using an advanced external beam radiotherapy technique, IMRT, for partial breast irradiation. In their study, the results were identical with our study, while the mean heart dose was 4.5 and 2 Gy in MIBT and IMRT respectively (P<0.05) and the ipsilateral lung was spared better with MIBT. The author concluded that the MIBT could generally spare the normal tissues and critical structures (except for heart) better compared to IMRT.
Further studies were recommended to take into account the location in the breast quadrant of the tumor bed and the anatomy of the OARs to decide whether modalities of treatment were suitable. Medially-located tumors may receive higher mean heart dose and LAD dose compared to laterally-located tumors. The relative position of the heart and LAD and even the cardiac size was also needed to be evaluated before treatment. Another difference should be taken into account was the different breast volumes between Asian and Caucasian women. Asian women generally have smaller breast volumes compared to Caucasian women. Due to smaller breast volume, the tumor bed of the breast was closer to the chest wall, hence resulting in higher dose to the heart and LAD.
There are some limitations in this study. First, this is a retrospective study in a single center, and the study population was small. Second, the CT images used for the digital phantom plan were the same as the ISBT plan, therefore the body contour and the patient position were not identical to the real-world clinical practice. Third, the catheters indwelling in the breast may draw the breast tissue away from the chest wall from its normal condition and the catheters in the breast may slightly change the tissue inhomogeneity of breast. Forth, in this study the alpha/beta of the heart was set as 3 Gy due to late response tissue. However, the variability in the alpha/beta may lead to a different result in biological equivalent dose. In larger fraction size of ISBT, the alpha/beta of 3 Gy may not be suitable, therefore the biological equivalent dose may be underestimated. These limitations could lead to underestimate the dosimetry of the digital phantom plans.
Conclusions
The results of this study provided several useful information of dosimetric comparison of OARs for left-sided breast cancer patients receiving APBI. Based on our findings, the mean heart dose of the ISBT and the PB-EBRT were all significantly lower than the WB-EBRT. Meanwhile, the mean dose to the ipsilateral lung was equivalent low in both APBI techniques. Further investigations were needed whether the dosimetric outcome was correlated with the clinical outcome of OARs treated by ABPI.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Pei-Wei Shueng, Yen-Wen Wu and Long-Sheng Lu) for the series “Cardio-Oncology” published in Therapeutic Radiology and Oncology. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tro.amegroups.com/article/view/10.21037/tro-21-27/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tro.amegroups.com/article/view/10.21037/tro-21-27/coif). The series “Cardio-Oncology” was commissioned by the editorial office without any funding or sponsorship. WSL serves as an unpaid editorial board member of Therapeutic Radiology and Oncology from June 2020 to May 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Kaohsiung Veterans General Hospital Institutional Review Board (VGHKS21-CT7-01) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [Crossref] [PubMed]
- Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087-106. [Crossref] [PubMed]
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987-98. [Crossref] [PubMed]
- Laugaard Lorenzen E, Christian Rehammar J, Jensen MB, et al. Radiation-induced risk of ischemic heart disease following breast cancer radiotherapy in Denmark, 1977-2005. Radiother Oncol 2020;152:103-10. [Crossref] [PubMed]
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 2015;314:1599-614. [Crossref] [PubMed]
- Katz A, Strom EA, Buchholz TA, et al. Locoregional recurrence patterns after mastectomy and doxorubicin-based chemotherapy: implications for postoperative irradiation. J Clin Oncol 2000;18:2817-27. [Crossref] [PubMed]
- Joo JH, Ki Y, Kim W, et al. Pattern of local recurrence after mastectomy and reconstruction in breast cancer patients: a systematic review. Gland Surg 2021;10:2037-46. [Crossref] [PubMed]
- Vicini FA, Cecchini RS, White JR, et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: a randomised, phase 3, equivalence trial. Lancet 2019;394:2155-64. [Crossref] [PubMed]
- Thomson DJ, Yom SS, Saeed H, et al. Radiation fractionation schedules published during the COVID-19 pandemic: a systematic review of the quality of evidence and recommendations for future development. Int J Radiat Oncol Biol Phys 2020;108:379-89. [Crossref] [PubMed]
- Polgár C, Van Limbergen E, Pötter R, et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009). Radiother Oncol 2010;94:264-73. [Crossref] [PubMed]
- Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO). Int J Radiat Oncol Biol Phys 2009;74:987-1001. [Crossref] [PubMed]
- Correa C, Harris EE, Leonardi MC, et al. Accelerated partial breast irradiation: executive summary for the update of an ASTRO evidence-based consensus statement. Pract Radiat Oncol 2017;7:73-9. [Crossref] [PubMed]
- Strnad V, Hildebrandt G, Pötter R, et al. Accelerated partial breast irradiation: 5-year results of the German-Austrian multicenter phase II trial using interstitial multicatheter brachytherapy alone after breast-conserving surgery. Int J Radiat Oncol Biol Phys 2011;80:17-24. [Crossref] [PubMed]
- Mehta LS, Watson KE, Barac A, et al. Cardiovascular Disease and Breast Cancer: Where These Entities Intersect: A Scientific Statement From the American Heart Association. Circulation 2018;137:e30-66. [Crossref] [PubMed]
- Chan TY, Tan PW, Tan CW, et al. Assessing radiation exposure of the left anterior descending artery, heart and lung in patients with left breast cancer: A dosimetric comparison between multicatheter accelerated partial breast irradiation and whole breast external beam radiotherapy. Radiother Oncol 2015;117:459-66. [Crossref] [PubMed]
- Shaitelman SF, Amendola B, Khan A, et al. American brachytherapy society task group report: Long-term control and toxicity with brachytherapy for localized breast cancer. Brachytherapy 2017;16:13-21. [Crossref] [PubMed]
- Smith BD, Bellon JR, Blitzblau R, et al. Radiation therapy for the whole breast: executive summary of an american society for radiation oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol 2018;8:145-52. [Crossref] [PubMed]
- Milo MLH, Offersen BV, Bechmann T, et al. Delineation of whole heart and substructures in thoracic radiation therapy: national guidelines and contouring atlas by the danish multidisciplinary cancer groups. Radiother Oncol 2020;150:121-7. [Crossref] [PubMed]
- Julian TB, Costantino JP, Vicini FA, et al. Early toxicity results with 3-d conformal external beam therapy (CEBT) from the NSABP B-39/RTOG 0413 accelerated partial breast irradiation (APBI) trial. Int J Radiat Oncol Biol Phys 2011;81:S7. [Crossref]
- Curigliano G, Cardinale D, Suter T, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol 2012;23:vii155-66. [Crossref] [PubMed]
- Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. 8th edition. Philadelphia: Wolters Kluwer Health, 2018.
- Amin MB, Edge SB, Greene FL, et al. editors. AJCC Cancer Staging Manual. 8th edition. New York: Springer International Publishing, 2017.
- Finnegan R, Lorenzen EL, Dowling J, et al. Analysis of cardiac substructure dose in a large, multi-centre danish breast cancer cohort (the DBCG HYPO trial): Trends and predictive modelling. Radiother Oncol 2020;153:130-8. [Crossref] [PubMed]
- Jacobse JN, Duane FK, Boekel NB, et al. Radiation dose-response for risk of myocardial infarction in breast cancer survivors. Int J Radiat Oncol Biol Phys 2019;103:595-604. [Crossref] [PubMed]
- Taylor C, Correa C, Duane FK, et al. Estimating the risks of breast cancer radiotherapy: evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J Clin Oncol 2017;35:1641-9. [Crossref] [PubMed]
- Taylor C, McGale P, Brønnum D, et al. Cardiac structure injury after radiotherapy for breast cancer: cross-sectional study with individual patient data. J Clin Oncol 2018;36:2288-96. [Crossref] [PubMed]
- Harris EE, Correa C, Hwang WT, et al. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol 2006;24:4100-6. [Crossref] [PubMed]
- Taylor CW, Kirby AM. Cardiac side-effects from breast cancer radiotherapy. Clin Oncol (R Coll Radiol) 2015;27:621-9. [Crossref] [PubMed]
- Strnad V, Ott OJ, Hildebrandt G, et al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: a randomised, phase 3, non-inferiority trial. Lancet 2016;387:229-38. [Crossref] [PubMed]
- Piroth MD, Baumann R, Budach W, et al. Heart toxicity from breast cancer radiotherapy: Current findings, assessment, and prevention. Strahlenther Onkol 2019;195:1-12. [Crossref] [PubMed]
- Nilsson G, Holmberg L, Garmo H, et al. Distribution of coronary artery stenosis after radiation for breast cancer. J Clin Oncol 2012;30:380-6. [Crossref] [PubMed]
- Major T, Stelczer G, Pesznyák C, et al. Multicatheter interstitial brachytherapy versus intensity modulated external beam therapy for accelerated partial breast irradiation: a comparative treatment planning study with respect to dosimetry of organs at risk. Radiother Oncol 2017;122:17-23. [Crossref] [PubMed]
Cite this article as: Chiang SW, Hsueh HP, Liu WS. Cardiac dosage comparison among whole breast irradiation and partial breast irradiation techniques. Ther Radiol Oncol 2022;6:3.


