
Cardiovascular implantable electronic devices may tolerate high dose radiotherapy: an updated case report with long term follow-up
IntroductionOther Section
Cardiovascular disease was common in the clinic and cardiovascular implantable electronic devices (CIED) was one of the modalities for patients with cardiovascular disease with rate issues. As cancer incidence and prevalence increased, more and more patients with CIED may need to be treated with radiotherapy. The radiotherapy dose commonly used in cancer treatment was high and it may be dangerous to delivery this high dose radiotherapy to CIED.
However, it was well known that radiotherapy may led to malfunction of CIED [either implantable cardiac pacemakers (ICP) or implantable cardioverter defibrillators (ICDs)] (1). An earlier report (TG-34) by American Association of Physicists in Medicine (AAPM) published in 1994 suggested to consider patients with CIED dose >2 Gy as high risk (2). An updated report (TG-203) published by AAPM in 2019 (1) had loosened the threshold for high risk as 5 Gy but it was still a concern for patients with CIED close to radiotherapy targets because curative radiotherapy often employed dose much higher than 5 Gy. We present the following case in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/tro-21-18).
Case presentationOther Section
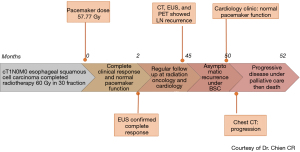
A case of cT1N0M0 esophageal squamous cell carcinoma with ICP was treated with radiotherapy. The patient information, clinical finding, and cancer treatment information as well as early follow-up result of ICP function had been described previously (3). In brief, he had pacing dependent left bundle branch block with congestive heart failure and received permanent ICP implantation (Medtronic Insync III 8042 with active leads of A lead Medtronic 5594-53 & V lead Medtronic 5076-58) in Aug 2009 as cardiac resynchronization therapy. He received curative intensity modulated external beam radiotherapy to esophageal tumor for 60Gy/30 fractions within Jun 2012–Jul 2012 using 6 MV X-ray in 400 MU/min. After he completed radiotherapy dose of 60 Gy in 30 fractions, the maximal ICP dose was 57.77 Gy in the lead whereas the maximal dose in the ICP generator was 0.39 Gy. He achieved complete clinical response of his esophageal squamous cell carcinoma 2 months after radiotherapy and received regular cancer follow with computed tomography and/or endoscope subsequently. He was also followed by cardiologists (lastly 49 months after radiotherapy) and ICP remained functioning well during follow. However, 45 months after radiotherapy, routine surveillance computed tomography (CT) with subsequent endoscopic ultrasound (EUS) plus biopsy and positron emission tomography (PET) (Figure 1, see the four images of PET) revealed regional and distal lymph nodes metastases without local recurrence over esophageal mucosa. He received best supportive care (BSC) thereafter by his preference under share care by palliative specialists then progressive disease was confirmed by CT 50 months after radiotherapy, then he died peacefully 52 months after high dose radiotherapy without clinical evidence of ICP malfunction clinically. The timeline of this patients was shown in Figure 2.
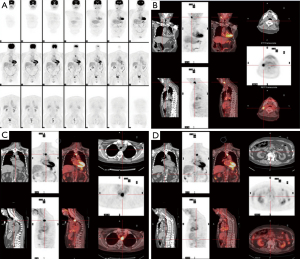
All procedures performed in studies involving human participants were in accordance with the ethical standards of the research ethics committee of our institute [CMUH106-REC3-119 (CR2)] and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
DiscussionOther Section
In the updated AAPM TG-203 report, higher than 5 Gy to CIED was considered as high risk of CIED malfunction though dose tolerance up to 20 Gy was also possible (1). Similar threshold (5 Gy) had also been recommended by the cardiology literatures (4). These dose cut-off points were used for risk classification but not as an upper limit for tolerable dose for CIED.
Possibly due to the concern of potential malfunction after high dose radiotherapy, there were few clinical studies available in the literatures. In the in vivo studies summarized in table IV of TG-203 (1), only two studies had reported patients treated with more than 50 Gy (5,6). Tsekos et al. reported a case of recurrent neuroendocrine carcinoma treated with radiotherapy of 50.4 Gy in 28 fractions. The pacemaker received full prescribed dose and no malfunction was noted during radiotherapy although decreased magnetic frequency of the pacemaker was noted. However, no follow-up data after radiotherapy was reported (5). Among the eight patients reported by Wadasadawala et al. (6), only one non-small cell lung cancer patient received 60 Gy in 30 fractions with pacemaker within radiation port and there were no malfunction of pacemaker within 9 months’ follow-up. The other seven patients had either received 39 Gy in 13 fraction or pacemaker out of radiation port. Therefore, our updated case reported provided clinical evidence that CIED may tolerance high dose radiotherapy after long term (more than 4 years) follow-up. However, our results should also be interpreted with caution because the Dmax to ICP generator was only 0.39 Gy in our patient whereas the high dose region (the lead of ICP) may tolerate high dose as mentioned in TG-203. Currently TG-203 stated “It is not known whether they (leads) should be considered as part of the CIED. The most practical option is therefore to include them until this issue is better understood.” On the contrary, in vitro studies had reported 32% malfunctioned device at dose ≤50 Gy (1). Therefore, clinical studies of larger sample size and longer follow-up were needed to clarify the real clinical risk when CIED received high radiotherapy dose.
ConclusionsOther Section
Our updated case report provided clinical evidence that CIED may tolerate high dose radiotherapy after long term (more than 4 years) follow-up which was rarely reported in the literatures. However, clinical studies of larger sample size and longer follow-up were needed to clarify the real clinical risk when CIED received high radiotherapy dose.
Take-away lesson: The lead of CIED may tolerate high dose radiotherapy after long term (more than 4 years) follow-up in some cases.
AcknowledgmentsOther Section
We thanked Mrs. Chia-Chin Li for her help in preparation of this manuscript.
Funding: This work was supported by China Medical University Hsinchu Hospital (CMUHCH-DMR-110-015).
FootnoteOther Section
Provenance and Peer Review: This article was commissioned by the Guest Editors (Pei-Wei Shueng, Yen-Wen Wu and Long-Sheng Lu) for the series “Cardio-Oncology” published in Therapeutic Radiology and Oncology. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/tro-21-18
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tro-21-18). The series “Cardio-Oncology” was commissioned by the editorial office without any funding or sponsorship. JAL serves as an unpaid editorial board member of Therapeutic Radiology and Oncology from Apr 2020 to Mar 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the research ethics committee of our institute [CMUH106-REC3-119(CR2)] and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Miften M, Mihailidis D, Kry SF, et al. Management of radiotherapy patients with implanted cardiac pacemakers and defibrillators: A Report of the AAPM TG-203†. Med Phys 2019;46:e757-88. [Crossref] [PubMed]
- Marbach JR, Sontag MR, Van Dyk J, et al. Management of radiation oncology patients with implanted cardiac pacemakers: report of AAPM Task Group No. 34. American Association of Physicists in Medicine. Med Phys 1994;21:85-90. [Crossref] [PubMed]
- Lai YL, Liang JA, Lin KH, et al. High Dose Radiotherapy of an Esophageal Cancer Patient with Cardiac Pacemaker Implantation-A Case Report. Therapeut Radiol Oncol 2013;20:235-42.
- Indik JH, Gimbel JR, Abe H, et al. 2017 HRS expert consensus statement on magnetic resonance imaging and radiation exposure in patients with cardiovascular implantable electronic devices. Heart Rhythm 2017;14:e97-e153. [Crossref] [PubMed]
- Tsekos A, Momm F, Brunner M, et al. The cardiac pacemaker patient--might the pacer be directly irradiated? Acta Oncol 2000;39:881-3. [Crossref] [PubMed]
- Wadasadawala T, Pandey A, Agarwal JP, et al. Radiation therapy with implanted cardiac pacemaker devices: a clinical and dosimetric analysis of patients and proposed precautions. Clin Oncol (R Coll Radiol) 2011;23:79-85. [Crossref] [PubMed]
Cite this article as: Lin KH, Hsu HS, Feng CL, Kao CH, Chou SH, Liang JA, Chien CR. Cardiovascular implantable electronic devices may tolerate high dose radiotherapy: an updated case report with long term follow-up. Ther Radiol Oncol 2021;5:18.


