
Melanoma brain metastases: the outcome of whole brain radiation therapy in the era of effective systemic therapy
IntroductionOther Section
Melanoma has a high propensity to metastasise into the brain in patients with advanced disease (1). Brain metastases are associated with poor prognosis and often result in deterioration in neurological function and quality of life and/or neurological death (2-4). The treatment approach to melanoma brain metastases (MBM) is multimodal including systemic therapy, often in combination with brain directed local therapy such as surgical resection and radiotherapy (RT).
The use of whole brain radiation therapy (WBRT) is declining due to the intracranial activity of systemic drug therapies in melanoma (targeted therapy and immunotherapy (5-7), increasing evidence for use of stereotactic radiosurgery (SRS) in multiple brain metastases (8), the lack of efficacy in the adjuvant setting (9), and the toxicity of WBRT (9). For patients who progress in the brain despite treatment with systemic agents or have symptomatic metastases at initial diagnosis of metastatic disease, local treatment options include surgery, SRS and WBRT. WBRT is generally recommended for patients with multiple brain metastases not suitable for surgery and/or SRS that have demonstrated some melanoma resistance to systemic therapy (10). In the past few years, at our institutions, selected patients received WBRT after multidisciplinary team discussion considering various patient and tumour factors. The use of WBRT must be balanced against the potential benefits and toxicity (9). Anecdotally, some patients have sustained response after WBRT. The aim of the study was therefore to report the outcome of WBRT in the era of systemic therapy with intracranial activity. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/tro-21-6).
MethodsOther Section
Study design, inclusion and exclusion criteria
This was a retrospective cohort study of patients from Melanoma Institute Australia who underwent WBRT for MBM at three centres (Mater Hospital, Westmead Hospital, Nepean Hospital) between 2011 and 2018. Patients were eligible if they underwent WBRT concurrently or after progression with immune checkpoint inhibitor (anti-CTLA4 inhibitor, anti-PD1 inhibitor) and/or BRAF/MEK targeted therapy. Patients who received no systemic treatment or who did not complete the whole course of WBRT were excluded. This study was approved by the institutional ethics committee (approval number MIA2019/262) and individual consent for this retrospective analysis was waived. The study was performed in accordance with the tenets set forth in the Declaration of Helsinki (as revised in 2013) and Good Clinical Practice guidelines.
Patient characteristics captured included Eastern Cooperative Oncology Group (ECOG) performance status, total number of brain metastases and BRAF mutation status. Additional treatment characteristics included surgical resection, SRS, and type of systemic therapies (BRAF inhibitor, anti-PD 1, ipilimumab, and none). The RT fractionation schedule and the use of hippocampal avoidance WBRT and simultaneous integrated boost to larger lesions technique were at the discretion of the treating radiation oncologists. Patients were followed clinically with the treating clinician at 3-weekly to 3-monthly intervals. In general, MRI of the brain and CT and/or PET of the body were performed at baseline and every three months following treatment. During follow-up, the proportion of intracranial failure was determined by MRI.
Statistical analysis
Patients’ characteristics were summarised using frequency and proportion or median and range as appropriate. The clinical outcomes investigated include intracranial failure and overall survival (OS). Intracranial failure was defined as intracranial progression determined by MRI. Follow up time and OS were measured from the first date of WBRT treatment until death or last follow up dates. Patients who did not experience the outcome were censored at their last follow-up date. Survival of patients during follow-up was described using Kaplan-Meier method stratified by group. Difference in survival between groups was assessed through the log-rank test.
To evaluate the impact of the current state-of-the-art systemic therapies individually, the cohort was furthermore divided into patients treated 2010–2015 and 2016–2018 and baseline and treatment characteristics as well as survival outcomes reported separately.
The following groups were considered: Concurrent WBRT and systemic therapy versus systemic therapy sequentially following completion of WBRT versus WBRT after drug failure in the brain (BRAF wild type vs. BRAF mutant tumor genotypes); prior neurosurgery (yes vs. no), prior SRS (yes vs. no), concurrent simultaneous integrated boost (yes vs. no), age (≤65 vs. >65 years), gender, leptomeningeal disease (yes vs. no), ECOG status (0–1 vs. ≥2), neurological symptoms (yes vs. no) and number of metastasis (1–3 vs. 4–9 vs. ≥10). Univariable and multivariable Cox regression were performed to evaluate the association between OS and baseline factors. The final multivariable model was obtained using a stepwise backward selection on the initial models that included fractionation schedule and variables with a P value <20% from the univariate analysis (11). Two-sided P values <0.05 were considered significant. All statistical analyses were performed using SPSS ver. 21 (IBM SPSS Statistics; IBM Corporation, Armonk, New York, USA), Prism Version 8 (GraphPad Software, Inc., San Diego, CA, USA) and R version 3.6.1 (R Core Team, Vienna, Austria).
ResultsOther Section
Patient characteristics
A total of 106 patients who underwent WBRT between 2011 and 2018 were identified. Patients who did not receive any systemic therapy (n=8) and those who did not complete WBRT due to clinical deterioration (n=8) were excluded. Thus, the final analysis included 90 patients (Table 1). The median age at the time of the diagnosis of brain metastases was 63.0 years (range, 31–84) and 59 patients (66%) were males. The majority had had an ECOG performance status 0 (26 patients, 29%) to 1 (47 patients, 52%). Fifty patients (56%) had neurological symptoms at the beginning of WBRT.
Table 1
| Characteristics | N (n=90) | % |
|---|---|---|
| Gender | ||
| Male | 59 | 66 |
| Female | 31 | 34 |
| Age at the diagnosis of MBM, years | ||
| Median [range] | 63 [31–84] | |
| <65 | 52 | 58 |
| ≥65 | 38 | 42 |
| ECOG performance status | ||
| 0 | 26 | 29 |
| 1 | 47 | 52 |
| 2 | 14 | 16 |
| 3 | 3 | 3 |
| BRAF mutation | ||
| Yes | 42 | 47 |
| No | 48 | 53 |
| Leptomeningeal disease | ||
| Yes | 21 | 23 |
| No | 69 | 77 |
| No. of metastases | ||
| 1 | 4 | 6 |
| 2–3 | 6 | 9 |
| 4–9 | 18 | 26 |
| ≥10 | 41 | 59 |
| Neurological symptoms | ||
| Yes | 50 | 56 |
| No | 40 | 44 |
MBM, melanoma brain metastases.
Twenty-one patients (23%) had leptomeningeal disease as well as parenchymal metastasis. Of the remaining 69 patients (77%), 41 patients (59%) had 10 or more MBM, while 18 patients (26%) had 4– 9 MBM and 10 patients (15%) had 1–3 MBM. Of the 10 patients with 1–3 MBM, nine (90%) had previous surgery or SRS to the brain metastases and WBRT was given in a salvage setting after progression or in adjuvant setting on a clinical trial (9), and one patient was unwell with neurological symptoms.
Treatment details
WBRT
The prescribed dose of the WBRT ranged from 20 to 30 Gy with a mean dose of 28.8 Gy and with median fractions of 10 (range, 5–15 fractions, Table 2). Seventy-nine patients (88%) received 30 Gy in 10 fractions. Twenty-eight patients (31%) received hippocampal-avoidance WBRT. Concurrent simultaneous integrated boost (SIB) with WBRT was given in 27 patients (30%) with a mean total dose of 43 Gy (range, 34–63 Gy) in median fraction of ten (range, 5–15 fractions).
Table 2
| Characteristics | Total(n=90) | Second procedure | Third procedure |
|---|---|---|---|
| Whole brain radiation therapy | |||
| Mean total dose, Gy [range] | 28.8 [20–30] | ||
| Median fractions [range] | 10 [5–15] | ||
| Neurosurgery | |||
| Before WBRT, No. | 28 (31%) | 2 (2%) | 1 (1%) |
| After WBRT, No. | 6 (7%) | 1 (1%) | 0 |
| Concurrent SIB with WBRT, No. | 27 (30%) | ||
| Median number of lesions [range] | 3 [1–10] | ||
| Mean SIB dose, Gy [range] | 43 [34–63] | ||
| Median fractions [range] | 10 [5–15] | ||
| Stereotactic radiosurgery | |||
| Before WBRT, No. | 15 (17%) | 5 (6%) | 1 (1%) |
| Median lesions [range] | 2 [1–3] | 1 [1–3] | 3 |
| Mean total dose, Gy [range] | 19.5 [14–27] | 21 [18–27] | 20 |
| Median fractions [range] | 1 [1–3] | 1 [1–3] | 1 |
| After WBRT, No. | 8 (9%) | 2 (2%) | |
| Median lesions [range] | 5 [1–40] | 1.5 [1–2] | |
| Mean total dose, Gy [range] | 19.5 [13–27] | 19 [18–20] | |
| Median fractions [range] | 1 [1–3] | 1 |
WBRT, whole brain radiation therapy.
Systemic therapy
A breakdown of systemic therapy in WBRT-treated patients is provided in Figure 1. Twenty patients (22%) had first-line systemic therapy concurrently with WBRT at the presentation of MBM. These patients had a median ECOG of 1 (range, 0–3). One patient had 1 MBM, four patients had 2–3 MBM and two patients had 4–9 MBM, while 13 patients had 10 or more MBM.
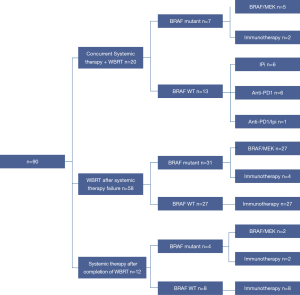
Fifty-eight patients (64%) progressed on systemic therapy in the brain prior to WBRT (Table 3) and had a median ECOG of 1 (range, 0–3). Nine patients (16%) did not have any further systemic therapy after WBRT, 49 patients (84%) had further systemic therapy. These included further immunotherapy in 38 patients (78%), targeted therapy in 10 patients (20%), and clinical trial participation in one patient (2%).
Table 3
| Variable | BRAF/MEK inhibitors | Anti-PD1 | Ipilimumab | Anti-PD1 and ipilimumab | Clinical trial | Total |
|---|---|---|---|---|---|---|
| BRAF mutant type (n=42) | ||||||
| Prior systemic therapy (1st line) | 27 | 2 | 0 | 2 | 0 | 31 |
| Prior systemic therapy (2nd line) | 4 | 5 | 3 | 3 | 0 | 15 |
| BRAF wild type (n=48) | ||||||
| Prior systemic therapy (1st line) | 0 | 15 | 8 | 4 | 0 | 27 |
| Prior systemic therapy (2nd line) | 0 | 4 | 2 | 2 | 1 | 9 |
WBRT, whole brain radiation therapy.
In 12 patients (13%), systemic therapy was administered sequentially following completion of WBRT. These patients had a median ECOG of 1 (range, 0–2) and two patients had one MBM, five patients had four to nine MBM, while five patients had 10 or more MBM.
Surgery and SRS
Fifteen patients (17%) had SRS prior to WBRT with a median number of treated lesions of two (range, 1–3 lesions), with a mean total dose of 19.5 Gy (range, 14–27 Gy) in median of one fraction (range, 1–3 fractions). Of those, five patients received a second course and one patient a third course of SRS. These patients had a median age of 61 and a median time between SRS and WBRT of five months (range, 0–22 months). Twenty-eight patients (31%, median age 63.5 years) underwent surgery of MBM before WBRT, while two patients had a second operation and one patient a third operation. The median time between surgery and WBRT was 1 month (range, 0–27 months). In all patients, surgery was performed for symptomatic disease.
Eight patients (9%, median age 63 years)) had SRS after WBRT with a median number of treated lesions of five (range, 1–40 lesions), and a mean total dose of 19.5 Gy (range, 13–27 Gy) in median of one fraction (range, 1–3 fractions). The median time between WBRT and subsequent SRS was 5 months. Two patients received a second course of SRS. Six patients (7%, median age 67.5 years) underwent surgery after WBRT (four for progressive MBM and two for radionecrosis) and one patient had a second operation. The median time between WBRT and surgery was 9 months (range, 2–22 months).
Outcomes
The median follow-up was 4.5 months (range 0–64). At the time of analysis, death was reported for 75 patients (83%). The median OS from diagnosis of MBM was eight months (range, 1–83 months) and the median OS from the beginning of WBRT was 5 months (range, 0–64 months). The 12 months OS from WBRT was 28% (Figure 2A). Details on WBRT timing and systemic therapy for 12-month survivors are provided in Figure S1. Fifty-three patients (59%) had a follow-up MRI, while the remaining patients had progressive disease. Twenty-eight of these patients (53%) showed progression of existing MBM or developed new intracranial lesions at a median time of 8 months.
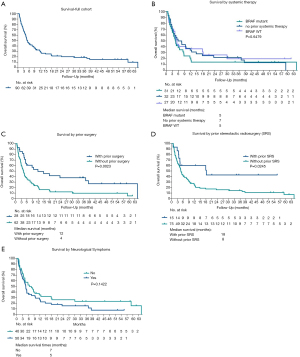
On univariate analysis, the presence of a BRAF mutation did not result in a statistically significant difference in median survival (P=0.41). Patients with BRAF mutant disease who had prior systemic treatment (n=31) had a median survival from WBRT of 4.6 months versus those with BRAF wild type disease (n=27) with a median survival of 5.2 months; patients with no systemic treatment prior to WBRT (n=32) had a median survival of 6.7 months. There was no significant difference between these three groups (P=0.65, Figure 2B). Prior brain directed local therapy (surgery or SRS) was associated with better survival. The median survival for those who had prior surgery was 12 months compared with four months for those who did not have prior surgery (P=0.0023, Figure 2C). Similarly, those who had prior SRS showed statistically significantly longer survival (18 vs. 8 months median survival time, P=0.0245, Figure 2D). Those who had neurological symptoms at the time of WBRT had poorer survival (median OS 5 vs. 7 months, Figure 2E).
In the multivariable analysis (Table 4), the presence of neurological symptoms was associated with worse OS from time of WBRT (P=0.029). Prior surgery remained significantly associated with better OS (HR 0.42, 95% CI: 0.24, 0.73, P=0.002) but not prior SRS (HR 0.51, 95% CI: 0.24, 1.08, P=0.079). WBRT with concurrent SIB did not improve the survival significantly (median survival 4 months without concurrent SIB versus 8 months with SIB, P=0.12). Factors such as age, gender, ECOG status, BRAF mutation status, number of MBM, and leptomeningeal disease did not influence the OS.
Table 4
| Variable | Univariable | Multivariable* | |||
|---|---|---|---|---|---|
| HR | P value | HR | P value | ||
| Age at diagnosis of MBM | |||||
| <65 | 1 | 0.9847 | |||
| ≥65 | 1.00 (0.63, 1.59) | ||||
| Gender | |||||
| Female | 1 | 0.1841 | |||
| Male | 0.73 (0.45, 1.16) | ||||
| Leptomeningeal disease | |||||
| No | 1 | 0.1461 | |||
| Yes | 1.48 (0.87, 2.49) | ||||
| ECOG | |||||
| 0–1 | 1 | 0.3876 | |||
| ≥2 | 1.29 (0.72, 2.32) | ||||
| Neurological symptoms | |||||
| No | 1 | 0.1447 | 1 | 0.0286 | |
| Yes | 1.4 (0.88, 1.41) | 1.72 (1.05, 2.78) | |||
| Number of MBM | |||||
| 1–3 | 1 | 0.0151 | |||
| 4–9 | 1.68 (0.63, 4.52) | ||||
| ≥2 | 2.96 (1.26, 6.94) | ||||
| Prior neurosurgery | |||||
| No | 1 | 0.0030 | 1 | 0.0020 | |
| Yes | 0.45 (0.26, 0.76) | 0.42 (0.24, 0.73) | |||
| Prior SRS | |||||
| No | 1 | 0.0288 | 1 | 0.0798 | |
| Yes | 0.44 (0.21, 0.92) | 0.51 (0.24, 1.08) | |||
| Prior systemic therapy | |||||
| No prior systemic therapy | 1 | 0.6512 | |||
| BRAF mutant with prior systemic therapy | 1.21 (0.71, 2.08) | ||||
| BRAF wild type with prior systemic therapy | 0.94 (0.53, 1.67) | ||||
*, derived using a backward model selection. Initial model included all variables with P value <0.20. WBRT, whole brain radiation therapy.
Impact of timing of systemic therapy
Kaplan-Meier plots, patient and treatment characteristics by the study period (2010–2015 vs. 2016–2018) are provided in Figure S2 and Tables S1-S3. There was no statistically significant difference in outcomes between the two study periods. However, patients treated 2010–2015 showed a significantly better ECOG status (ECOG 2–3, 9%) than patients treated 2016–2018 (ECOG 2–3, 32%, P=0.006).
DiscussionOther Section
Multidisciplinary team management of patients with melanoma is recommended by expert bodies (10,12,13). The management of MBM is carefully discussed by a melanoma multidisciplinary team including medical oncologists, radiation oncologists and neurosurgeons. In this selected cohort of 90 patients who were recommended to have WBRT in the era of systemic therapy with known intracranial activity, the median survival was 8 months from the initial diagnosis of MBM. Our study resulted in several main findings relevant for clinical practice in the management of MBM. Patients who underwent prior surgery or had no neurological symptoms demonstrated significantly better survival. On the other hand, the BRAF status and the type of systemic therapy prior to WBRT did not significantly influence survival. In the 21 patients who had leptomeningeal disease, they did not appear to have a worse survival compared to those with parenchymal MBM.
The OS of this cohort is favourable compared to studies prior to the availability of immunotherapy and targeted therapy, reporting a median OS in the range of 4–6 months after the diagnosis of MBM (3,14-16). Despite 64% of patients having progressed in the brain on BRAF/MEK inhibitors and/or immune checkpoint inhibitors prior to WBRT, the median OS was 5 months and the 12-month survival rate was 28% after WBRT. In multivariate analysis, prior neurosurgery was statistically significantly associated with better OS. Neurosurgery is generally indicated in patients with symptomatic MBM due to dominant lesions and in patients with better performance status and lower burden of intracranial and extracranial disease. In a previous study of mixed histologies, concomitant SRS was associated with improved survival over WBRT alone with a median survival time of 6.5 versus 4.9 months (17). Kocher et al. reported no statistically significant impact of adjuvant WBRT versus observation in patients treated with prior surgery or SRS with a median survival of 10.9 versus 10.7 month (18). Both studies included only patients with one to three brain metastases and mainly patients with lung and breast cancer (where drugs have little intracranial activity), with only a few patients with melanoma. In our study, the majority of patients had more than three or even more than 10 brain metastases and received WBRT as a last-line palliative therapy. On the other hand, surgery and SRS were associated with better survival, but those patients who had no prior surgery or SRS presumably had either brain metastasis too extensive for SRS or surgery, or poorer performance status, which may explain the worse survival in those who did not have prior surgery or SRS.
The indication for WBRT in combination with other modalities is complex, and must be considered in the light of recent advances in systemic therapies that can be effective in patients with MBM. For asymptomatic patients, ipilimumab has shown an intracranial response rate of 5–16% (19) and pembrolizumab or nivolumab alone achieved a response rate of 21–22%, and 46–56% with the combination of ipilimumab and nivolumab (6,20). BRAF inhibitors are also known to be effective in MBM with intracranial response rates of 39% for dabrafenib and 29% for vemurafenib alone (21,22), while the combination of the BRAF inhibitor dabrafenib and the MEK inhibitor trametinib achieved response rates of 58% 5. Therefore, in patients with asymptomatic, untreated MBM, systemic therapy (particularly immunotherapy) can be considered as first line treatment (as an alternative to local brain therapy) with multidisciplinary support from a radiation oncologist and a neurosurgeon.
Our study adds outcome data of a large cohort of patients with MBM treated with WBRT in addition to systemic therapies. Our results indicate that patients with BRAF mutant disease receiving targeted therapy prior to WBRT had similar survival outcomes compared to patients with BRAF wild type receiving prior immunotherapy. Previous studies have investigated the combination of ipilimumab and WBRT mainly in the setting of safety evaluations (23-26) and showed similar survival results in the range of 3.1–8.5 months, compared to our study. Regarding targeted therapy and WBRT, prior research showed median survival times of 4.6 months (27) which is also in line with our results. Concerning the timing of WBRT in relation to systemic therapy, patients receiving systemic treatment after WBRT had a slightly improved, but not statistically significantly better OS of 6.7 months. In prior studies, patients receiving systemic therapy initially at the start of or shortly after radiation treatment showed an additive effect of radiation therapy and systemic therapy (26,28-31) which was however not the case in our study. This discrepancy could be explained by the fact that prior studies used SRS instead of WBRT, which may trigger additional anti-tumor immune response.
It is important to note that medical therapy of MBM is an actively evolving field that has changed substantially in the recent years. In particular, systemic therapy with intracranial activity such as the combination of ipilimumab and anti-PD1 only became routinely available outside clinical trial setting in Australia in 2017. It is therefore not surprising to note that, when analysed separately, the cohort of patients treated from 2016 onwards had a significantly worse baseline ECOG performance status than patients treated earlier. In this era of modern systemic therapy, WBRT was restricted to patients with aggressive disease and/or who had already progressed previously. With state-of-the-art treatment, these patients with unfavourable prognosis had similar outcome after WBRT compared to patients with a more favourable disease condition several years ago.
Our main limitations were the retrospective design of our study, conferring risk of selection bias, and the lack of subgroup analysis regarding specific types of systemic treatments due to small numbers. A further limitation includes the lack of standardized reporting of toxicity of WBRT such as neurocognitive decline or radionecrosis.
In conclusion, in patients with MBM treated with systemic therapy with known intracranial activity, WBRT was used in our cohort in addition to surgery and SRS in selected patients. Future studies in a prospective randomized design should determine toxicity, quality of life and the sequencing of systemic treatment with RT in patients with MBM, particularly in patients with multiple brain metastases.
AcknowledgmentsOther Section
Funding: None.
FootnoteOther Section
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/tro-21-6
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tro-21-6). GVL received Salary support from NHMRC fellowship and the University of Sydney Medical Foundation, travel support from Bristol Myers Squibb, Novartis, Roche, Amgen, Pierre Fabre, MERCK and Array. She also received Consultancy fees from Bristol Myers Squibb, Novartis, Roche, Amgen, Pierre Fabre, MERCK and Array. MSC received Travel support and registration fees from Bristol Myers Squibb & MSD and honorarium from Bristol Myers Squibb, MSD, Novartis. AMM received Salary support from Cancer Institute NSW fellowship and Melanoma Institute Australia, travel support from Bristol Myers Squibb and consultancy fee from Bristol Myers Squibb, MSD, Novartis, Roche, Pierre Fabre. AMH received consultancy fee from Bayer and Provectus. She is a committee member for the Cooper Rice-Brading Foundation and a director of the Australia and New Zealand Sarcoma Association. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee (approval number MIA2019/262) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Jakob JA, Bassett RL Jr, Ng CS, et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer 2012;118:4014-23. [Crossref] [PubMed]
- Chiarion-Sileni V, Guida M, Ridolfi L, et al. Central nervous system failure in melanoma patients: results of a randomised, multicentre phase 3 study of temozolomide- and dacarbazine- based regimens. Br J Cancer 2011;104:1816-21. [Crossref] [PubMed]
- Davies MA, Liu P, McIntyre S, et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer 2011;117:1687-96. [Crossref] [PubMed]
- Fife KM, Colman MH, Stevens GN, et al. Determinants of Outcome in Melanoma Patients With Cerebral Metastases. Journal of Clinical Oncology 2004;22:1293-300. [Crossref] [PubMed]
- Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinib in patients with BRAF(V600)-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol 2017;18:863-73. [Crossref] [PubMed]
- Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. The Lancet Oncology 2018;19:672-81. [Crossref] [PubMed]
- Tawbi HA-H, Forsyth PAJ, Hodi FS, et al. Efficacy and safety of the combination of nivolumab (NIVO) plus ipilimumab (IPI) in patients with symptomatic melanoma brain metastases (CheckMate 204). J Clin Oncol 2019;37:9501. [Crossref]
- Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 2014;15:387-95. [Crossref] [PubMed]
- Hong AM, Fogarty GB, Dolven-Jacobsen K, et al. Adjuvant Whole-Brain Radiation Therapy Compared With Observation After Local Treatment of Melanoma Brain Metastases: A Multicenter, Randomized Phase III Trial. J Clin Oncol 2019;37:3132-41. [Crossref] [PubMed]
- Hong AM, Waldstein C, Shivalingam B, et al. Management of melanoma brain metastases: Evidence-based clinical practice guidelines by Cancer Council Australia. Eur J Cancer 2021;142:10-7. [Crossref] [PubMed]
- Cox DR. Regression Models and Life-Tables. Journal of the Royal Statistical Society Series B (Methodological) 1972;34:187-220. [Crossref]
- Marsden JR, Newton-Bishop JA, Burrows L, et al. Revised UK guidelines for the management of cutaneous melanoma 2010. J Plast Reconstr Aesthet Surg 2010;63:1401-19. [Crossref] [PubMed]
- Thompson JF, Williams GJ. Multidisciplinary care of cancer patients: a passing fad or here to stay? ANZ J Surg 2019;89:464-5. [Crossref] [PubMed]
- Raizer JJ, Hwu WJ, Panageas KS, et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol 2008;10:199-207. [Crossref] [PubMed]
- Staudt M, Lasithiotakis K, Leiter U, et al. Determinants of survival in patients with brain metastases from cutaneous melanoma. Br J Cancer 2010;102:1213-8. [Crossref] [PubMed]
- Vecchio S, Spagnolo F, Merlo DF, et al. The treatment of melanoma brain metastases before the advent of targeted therapies: associations between therapeutic choice, clinical symptoms and outcome with survival. Melanoma Res 2014;24:61-7. [Crossref] [PubMed]
- Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665-72. [Crossref] [PubMed]
- Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 2011;29:134-41. [Crossref] [PubMed]
- Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol 2012;13:459-65. [Crossref] [PubMed]
- Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 2016;17:976-83. [Crossref] [PubMed]
- Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:1087-95. [Crossref] [PubMed]
- McArthur GA, Maio M, Arance A, et al. Vemurafenib in metastatic melanoma patients with brain metastases: an open-label, single-arm, phase 2, multicentre study. Ann Oncol 2017;28:634-41. [Crossref] [PubMed]
- Williams NL, Wuthrick EJ, Kim H, et al. Phase 1 Study of Ipilimumab Combined With Whole Brain Radiation Therapy or Radiosurgery for Melanoma Patients With Brain Metastases. Int J Radiat Oncol Biol Phys 2017;99:22-30. [Crossref] [PubMed]
- Gerber NK, Young RJ, Barker CA, et al. Ipilimumab and whole brain radiation therapy for melanoma brain metastases. J Neurooncol 2015;121:159-65. [Crossref] [PubMed]
- Gabani P, Fischer-Valuck BW, Johanns TM, et al. Stereotactic radiosurgery and immunotherapy in melanoma brain metastases: Patterns of care and treatment outcomes. Radiother Oncol 2018;128:266-73. [Crossref] [PubMed]
- Silk AW, Bassetti MF, West BT, Tsien CI, Lao CD. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med 2013;2:899-906. [Crossref] [PubMed]
- Gupta A, Roberts C, Tysoe F, et al. RADVAN: a randomised phase 2 trial of WBRT plus vandetanib for melanoma brain metastases - results and lessons learnt. Br J Cancer 2016;115:1193-200. [Crossref] [PubMed]
- Cohen-Inbar O, Shih HH, Xu Z, Schlesinger D, Sheehan JP. The effect of timing of stereotactic radiosurgery treatment of melanoma brain metastases treated with ipilimumab. J Neurosurg 2017;127:1007-14. [Crossref] [PubMed]
- Mastorakos P, Xu Z, Yu J, et al. BRAF V600 Mutation and BRAF Kinase Inhibitors in Conjunction With Stereotactic Radiosurgery for Intracranial Melanoma Metastases: A Multicenter Retrospective Study. Neurosurgery 2019;84:868-80. [Crossref] [PubMed]
- Olson AC, Thomas S, Qin R, et al. Outcomes and toxicity of stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab. Melanoma Manag 2016;3:177-86. [Crossref] [PubMed]
- Schmidberger H, Rapp M, Ebersberger A, et al. Long-term survival of patients after ipilimumab and hypofractionated brain radiotherapy for brain metastases of malignant melanoma: sequence matters. Strahlenther Onkol 2018;194:1144-51. [Crossref] [PubMed]
Cite this article as: Waldstein C, Wang W, Lo S, Shivalingam B, Fogarty GB, Carlino MS, Menzies AM, Long GV, Hong AM. Melanoma brain metastases: the outcome of whole brain radiation therapy in the era of effective systemic therapy. Ther Radiol Oncol 2021;5:11.


