
The volume of low-dose thoracic irradiation influences systemic inflammation-immunity status after chemoradiation in esophageal cancer
Introduction
For inoperable locally advanced disease, concurrent chemoradiotherapy (CCRT) used according to the Radiation Therapy Oncology Group 85-01 protocol is the standard therapy (1). Although ionizing radiation or radiation therapy (RT) is mandatory for local control, the lung and heart are two critical organs that are impacted during thoracic radiation. A majority of the thoracic volume is irradiated especially during intensity-modulated radiotherapy, although techniques involving the use of multiple fields during RT expose the lungs and heart to a low dose of radiation (2). Radiation dose-volume effects in the lung, such as radiation pneumonitis, are well known (3,4), and radiation dose-volume may also independently impact survival outcomes (5-7). Cardiac irradiation is also correlated with heart disease, especially ischemic events, and may occur earlier than historically understood (8-11). There is also evidence correlating the dosage of cardiac irradiation with survival (12,13). In addition to affecting the gross tumor and organs, radiation also impacts the adjacent microenvironment (14,15); immune cells surrounding the gross tumor, stromal cells, and adjacent vasculature are also affected by different doses of RT (16).
Lymphopenia and prognosis have been established to have a clinical correlation (17). A model has been proposed to estimate the effective dose of RT experienced by circulating immune cells after administration of CCRT for esophageal cancer, and it also provides the mean lung dose, mean heart dose, mean liver dose, and integral dose of the scanned body region. It demonstrated the correlation between a higher effective dose and grade 4 lymphopenia (18). Although lymphopenia is also triggered by cytotoxic chemotherapy, it is commonly seen after the administration of RT and is associated with the recurrence of and mortality in several solid tumors (19). Although the dose of cardiac irradiation is associated with immunosuppression and even poor survival in patients with lung cancer (12), the exact correlation between different doses and pathological changes in each organ, especially the lung, which is the largest thoracic organ, and the heart, which circulates the entire blood volume, during administration of CCRT for esophageal cancer has not been reported.
Systemic inflammation-immunity, evaluated in terms of the neutrophil-lymphocyte ratio (NLR), has been correlated with prognosis in esophageal cancer (20-22). NLR is an easy tool to understand inflammation-immunity dynamics at various time periods. We hypothesized that the volume of the two largest organs irradiated during thoracic irradiation contributes to changes in general inflammation-immunity conditions. The heart also circulates the entire volume of blood, and therefore, circulating immune cells are also irradiated during treatment. Lymphocytes are most vulnerable to irradiation (23,24) and are critical anti-tumor immune cells (25,26); therefore, we hypothesized that the volume of irradiated heart may affect lymphocyte counts during treatment. In this study, we investigate the association between survival outcomes and inflammation and determine the possible correlation between patient characteristics, during the pretreatment and the treatment period, and relevant dosimetric parameters. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tro-20-62).
Methods
Patient characteristics and study design
We retrospectively selected 93 patients from the Changhua Christian Hospital with non-metastatic thoracic esophageal cancer, who were treated with non-surgical treatments including definitive RT with or without induction, concurrent, and adjuvant chemotherapy. All patients were diagnosed between January 2010 and December 2015. Patients who had received a complete course of RT, with a median total radiation dose of 59.4 (range: 48.6–72) Gy and standard daily fractionation (1.8–2.0 Gy per fraction), were included in the study, and they had undergone complete blood count (CBC) tests during different periods of interest. All patients had esophagogastroduodenoscopy (EGD) biopsy-proven squamous-cell carcinoma, and we directly measured the gross tumor size under the scope. We used the 7th edition of the Union for International Cancer Control/American Joint Committee on Cancer TNM classification system for staging using chest CT scans. Bronchoscopy was optionally used to evaluate the trachea if there was any suspicion of direct invasion based on the chest CT scan. A metastatic survey with whole-body F-18 fluorodeoxyglucose positron emission tomography (PET)/computed tomography (CT), Tc99m methylene diphosphonate bone scan, or abdominal sonography was also used, if it was part of the initial or further follow-up workup based on the physician’s decision. Patients with any other cancer that was diagnosed or treated before this cancer, and those with synchronous cancer, were excluded. The age-adjusted Charlson comorbidity index (ACCI) score (27,28) was calculated (current esophageal cancer diagnosis not included) to estimate the 10-year pre-treatment risk of mortality. Treatment-related toxicities were graded using the Common Terminology Criteria for Adverse Events, version 4.0. After treatment, we used chest CT scans and EGD every 3–6 months to evaluate local, regional, and distant recurrences, in combination with the study of any other metastatic disease if needed.
Patient follow-up was updated and censored on February 29, 2020. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Changhua Christian Hospital (CCH IRB No. 180310), and individual consent for this retrospective analysis was waived because the research presented no more than minimal risk.
Radiation treatment and dosimetric analysis
The target volume for RT consisted of the primary tumor, lymphadenopathy, and an additional 1-centimeter circumferential margin and a 3- to 5-centimeter longitudinal margin. Elective nodal irradiation is also be included in the target volume based on the physician’s discretion. The target volume and critical organ at risk, such as the heart and lungs, were reviewed and recontoured (if needed) without adding a margin to each organ. Further dosimetric analysis was performed by a dosimetrist and reviewed by a physician. The analysis was performed on all patients, with available RT plans on the pinnacle treatment planning system (Pinnacle Treatment Planning/Philips Radiation Oncology Systems, Fitchburg, WI, USA). The dose-volume histogram parameter was identified as Vx (%) because the heart and lungs received a dose of RT relative to their percent volume for at least x (Gy). The mean dose was also evaluated.
Chemotherapy regimen
Patients received induction, sequential, or concurrent chemotherapy with radiotherapy based on the patients’ general condition and physician’s discretion. Concurrent chemotherapy was strongly recommended, and patients were provided sequential chemotherapy only if they exhibited poor performance with concurrent chemotherapy. The chemotherapy regimen involved intravenous administration of 75 mg/m2 of cisplatin on the first day followed by the continuous infusion of fluorouracil, 1,000 mg/m2 in the next four days in each session. Chemotherapy was repeated every 4 weeks for four cycles, and if patients exhibited a creatinine clearance rate <60 mL/min, carboplatin was used instead of cisplatin. CCRT with weekly cisplatin was only performed if the patient could not tolerate the above regimen. One to two cycles of induction chemotherapy, consisting of the same regimen as the concurrent chemotherapy, was used to reduce disease burden before initiation of radiation.
Hematological parameters
We estimated baseline absolute neutrophil count (ANC) and absolute lymphocyte count (ALC) before the commencement of any treatment, including induction chemotherapy or CCRT. The ALC nadir was determined to be the lowest ALC recorded during RT. NLR refers to the ratio of ANC to ALC, and this was recorded prior to the commencement of treatment, as the baseline NLR (NLR-b), and for the highest NLR during CCRT (NLR-h), i.e., the day with the lowest ALC during RT.
Statistical analysis
Data continuously recorded are presented as median and range, whereas categorical data are presented as numbers and percentages. Clinical endpoints consisted of overall survival (OS), progression-free survival (PFS), disease-specific survival (DSS), freedom from distant metastasis (FFDM), and freedom from locoregional recurrence (FFLR). Follow-up time and time of recording clinical endpoints were calculated from the date of diagnosis. OS is defined as the time until death. PFS is defined as time until the patient is clinically or radiologically suspected to have encountered locoregional recurrence or distant metastasis or death, whichever came first. DSS is defined as death from tumor progression or related complications. FFDM or FFLR is defined as the time until distant metastasis or locoregional recurrence, respectively. Patients who did not experience locoregional recurrence or distant metastasis were censored at the date of the last follow-up.
A Cox regression model was used to analyze the relationship between NLR-b with OS and NLR-h with OS. The median NLR-b (3.68) was chosen as the threshold to dichotomize continuous numerical data and to increase specificity. OS, PFS, DSS, FFDM, and FFLR rates were estimated using Kaplan-Meier analyses by log-rank test to calculate the significance of survival estimate differences. To test the possible relationships between the NLR-b and clinical variables, we used Spearman’s rank correlation coefficient. A multivariate logistic regression model was used to analyze the correlations between variables, identified using Spearman’s rank correlation, and NLR-b. In addition, we used the Pearson correlation coefficient to find a possible correlation of NLR-h and decreased ALC percentage with dosimetric parameters of the normal organ. A P value of ≤0.05 was considered significant. Hazard ratios and odds ratios were reported with a 95% confidence interval (CI). All tests were performed using IBM® SPSS®, version 26 (SPSS IBM, Armonk, NY, USA).
Results
Treatment modalities, patient outcome, and cause of death
Patient and tumor characteristics are summarized in Table 1, and the treatment modalities are summarized in Table 2. Most patients underwent a PET/CT (74%) as part of their initial staging and workup. All patients were diagnosed with squamous-cell carcinoma, and majority of them had grade II squamous-cell carcinoma (69%). Various radiation techniques were used, consisting of either three-dimensional conformal RT (3D-CRT) (23.7%), intensity-modulated RT (IMRT) (43%), or volumetric-modulated arc therapy (33.3%). More than one-quarter (27.9%) of patients also received online cone-beam CT-based imaging correction, such as image-guided RT, before treatment. The median prescribed dose was 59.4 (range: 48.6–72) Gy.
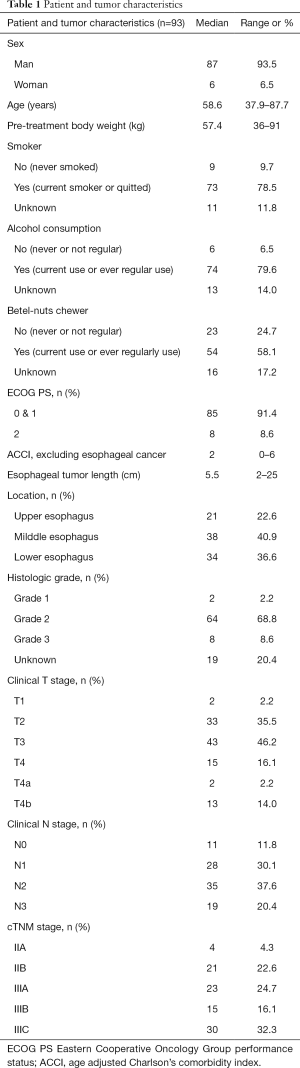
Full table
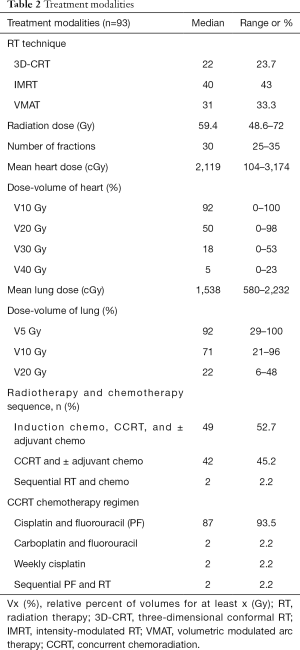
Full table
Almost all patients (97.9%) received concurrent chemotherapy with RT, and only two patients received chemotherapy and RT sequentially. More than half (52.7%) of the patients received induction chemotherapy before CCRT. The chemotherapy regimen comprised triweekly administration of cisplatin and fluorouracil (96%). Patients received induction chemotherapy (range: 0–2 cycle, median: 1 cycle) and concurrent chemotherapy with radiotherapy (range: 0–2 cycle, median: 1 cycle), based on their tolerance and the chosen chemotherapy regimen. The median cumulative cisplatin dose during and before completion of CCRT was 75 (range: 0–225) mg/m2 and 135 (range: 0–300) mg/m2, respectively.
During CCRT, the most acute toxicity of grade ≥3 was hematological toxicity, which was exhibited separately, from the other grade 3 toxicities, including dysphagia (6%), mucositis (2%), anorexia (1%), and fatigue (1%), and there was no reported grade ≥4 toxicity.
The median follow-up duration was 13 (range: 3–104) months in all patients and 61 (range: 53–104) months in survivors. In all patients, the estimated median OS was 13 (95% CI: 10.38–15.63) months, DSS was 14 (95% CI: 11.60–16.40) months, and PFS was 9 (95% CI: 7.70–10.30) months. The estimated 2-year FFDM and FFLR were 37.1% and 40.3%, respectively.
By the last follow-up, 83 patients (89%) died, wherein most patients (92%) died owing to disease progression or subsequent complications, while others died because of second primary cancer (5%), tuberculosis infection (1%), or due to unknown etiology (2%). Cardiac complications along with acute myocardial infarction or life-threatening arrhythmia were noted in four of the expired patients (5%), and no survivors reported newly diagnosed cardiac disease.
Hematological parameters and toxicity
Based on pre-treatment CBCs (baseline) of 87 patients, the median number of days between baseline CBC and initiation of RT was 18 days (range: 2–81). Median baseline ANC, ALC, and NLR were 5,384 (range: 1,716–14,309) cells/mm3, 1,635 (range: 512–4,127) cells/mm3, and 3.68 (range: 0.77–13.92), respectively. Thirteen out of 87 (14%) patients exhibited a low baseline ALC, wherein eight patients had grade 1 lymphopenia and five patients had grade 2 lymphopenia.
The day of ALC nadir and NLR-h was available for all patients. We found that ALC nadir and NLR-h occurred on the median day of 28 (range: 7–74 and 1–74, respectively) after initiation of RT, although 24 patients showed different days of ALC nadir and NLR-h. The median nadir ALC and NLR-h was 212 (range: 16–742.1) cells/mm3 and 16.8 (range: 2.64–155), respectively. Ten out of 93 patients (10.8%) had grade 2 lymphopenia, 41 out of 93 patients (44.1%) had grade 3 lymphopenia, and 42 out of 93 patients (45.1%) had grade 4 lymphopenia. The median decreased ALC percentage (%) from baseline to nadir was 86.18 (range: 53.25–98.76).
High NLR-b and NLR-h during CCRT are associated with worse OS
The Cox regression model was used to analyze the impact of NLR-b and NLR-h on OS. When NLR is regarded as a continuous variable, a higher NLR-b (HR: 1.109, 95% CI: 1.016–1.210, P=0.02) and NLR-h (HR: 1.007, 95% CI: 1.000–1.014, P=0.037) were significantly associated with worse OS. In addition, both higher NLR-b and NLR-h were significantly associated with worse PFS, DSS, and FFDM, but not FFLR (Table 3).

Full table
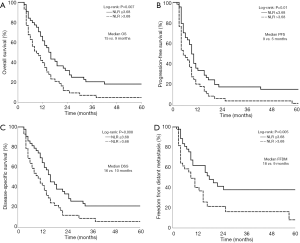
Kaplan-Meier analysis was used for evaluating the effect of dichotomized NLR-b values on OS. The median OS was stratified to 9 and 15 months when NLR-b >3.68 and ≤3.68, respectively (P=0.007, Figure 1A). The estimated 2-year OS rates was stratified to 11.6% and 31.8% when NLR-b >3.68 and ≤3.68, respectively. NLR-b values >3.68 are also associated with worse PFS (P=0.010), DSS (P=0.008), and FFDM (P=0.005), but not with FFLR (P=0.432, Figure 1B,C,D).
Patient characteristics associated with higher NLR-b
We used the Spearman’s rank correlation coefficient of NLR-b to test possible relationships between NLR-b and clinical variables (Table 4). Baseline body weight (Spearman’s correlation coefficient r=–0.251, P=0.019), primary esophageal tumor length (Spearman’s correlation coefficient r=0.324, P=0.011), and advanced clinical T stage (Spearman’s correlation coefficient r=0.230, P=0.032) were found to be significantly correlated with NLR-b >3.68. Using multivariate logistic regression, we found that primary esophageal tumor length (OR =1.345, P=0.021) was associated with a higher NLR-b (Table 5).
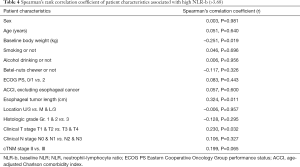
Full table

Full table
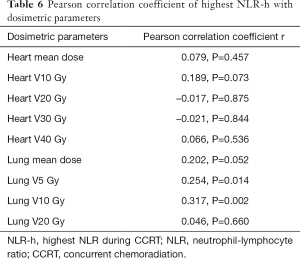
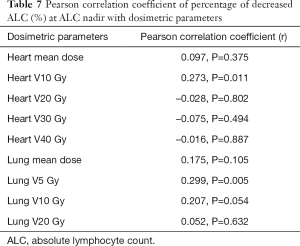
Dosimetric parameters are associated with NLR-h and percentage of decreased ALC (%) at ALC nadir
The relationship between NLR-h and continuous normal organ dosimetric parameters during CCRT is summarized in Table 5. The volume of low lung dose, such as lung V5 (Pearson correlation coefficient, r=0.254, P=0.014) and lung V10 (Pearson correlation coefficient, r=0.317, P=0.002), was found to be correlated with NLR-h (Table 6).
Full table
The percentage of ALC decrease at ALC nadir during CCRT was found to be significantly correlated with heart V10 (Pearson correlation coefficient, r=0.273, P=0.011) and lung V5 (Pearson correlation coefficient, r=0.299, P=0.005) (Table 7).
Full table
Discussion
In this retrospective study on thoracic esophageal cancer, we report that systemic inflammation at baseline and during RT were predictive of FFDM, DSS, PFS, and OS. Our findings suggest that gross tumor length is correlated with baseline systematic inflammation. In addition to irradiation of the gross tumor, the volume of the lungs that receives a low dose of radiation may further contribute to systemic inflammation. We also correlated the cardiac and lung volume exposed to low doses of irradiation with the extent of decrease in ALC during RT.
The interaction between tumor, host, and microenvironment has been reported to have a significant effect on immunotherapy outcomes (29). Neutrophils potentially promote cancer progression (30), and lymphocytes play a key role in mediating tumoricidal effects (26). NLR has been used as valuable biomarker to predict the survival outcomes of patients with esophageal cancer, regardless of whether they were treated surgically or with RT (20-22,31,32). We determined the OS correlation with NLR-b and NLR-h, and found the optimal cut-off value for NLR-b is 3.68. Interestingly, only FFLR was not correlated to NLR-b, indicating that NLR-b is more reflective of the systemic disease status. We also found that the initial tumor size is an indicator of a higher NLR-b, and the primary tumor size contributes to inflammatory-immunity dynamics. Therefore, a larger tumor size will further elicit a stronger inflammatory reaction and suppression of patient immunity. This correlation has also been demonstrated in thyroid cancer (33) and should be further investigated for a translational significance.
Dynamic changes in NLR during treatment are also potentially associated with survival outcomes and may be more informative than static baseline values (34,35). RT may turn so-called “cold” tumors “hot” via the release of pro-inflammatory mediators and increase in tumor-infiltrating immune cells (36). Thus, changes in NLR during treatment may serve as an early biomarker to reflect the treatment response and guide further treatment. We also demonstrate that the volume of lungs, such as V5 and V10, that receives low-dose irradiation is correlated with NLR-h, although cardiac volume receiving low dose irradiation is not correlated to NLR-h. This suggests that a larger volume of lung irradiated with low-dose RT induces a greater degree of systemic inflammation and suppression of immunity. Thus, strategies aimed to reduce NLR-h should be investigated further.
Recently, radiation-induced lymphopenia has also drawn attention, as survival outcomes have been correlated with lymphocyte nadir (17), and there is emerging evidence linking thoracic radiation dose to lymphopenia and survival outcomes (12,18,37,38). Although lymphotoxic chemotherapeutic agents exist, lymphocytes remain the most radiosensitive cell type in the body and are the only non-dividing cells killed by small doses of X-rays (23,24), and thus, radiation can exert immunosuppressive effects. Prior in vitro assays revealed that even a single small dose of 1 Gy can deplete and induce lymphoid cell death, while 2 Gy dose reduces the population of lymphocytes by 50% (39-41). Unintentional irradiation of the circulating lymphocyte pool causes lymphopenia (18). We found that the heart V10 and the lung V5 correlated with a decreased percentage of ALC at ALC nadir, and this is compatible with findings of the previous studies (37,42). We speculate that this is caused by a prolonged exposure of thoracic organs, especially the heart, and the circulating pool of blood to low-dose RT contributes to the ALC nadir. Irradiation of large vascular volumes causes lymphopenia, and the decrease in lymphocyte counts was directly proportional to the strength of irradiation (19). Although there exists a robust model to predict lymphopenia (18), here, we correlated the decrease in lymphocyte counts with low-dose thoracic irradiation simply by examining the two most relevant organs in the thorax. It is a convenient way for clinicians to evaluate treatment plans and predict the possibility of radiation-induced lymphopenia. Retrospective studies have reported a correlation between proton therapy and less severe treatment-related lymphopenia compared with photon therapy (43,44).
Our single-institute retrospective study has some limitations. We examined a small number of patients, who did not exhibit expression of other inflammatory markers, such as lactate dehydrogenase or C-reactive protein. Although we analyzed the pretreatment and nadir parameters during CCRT, the influence of different chemotherapy regimens on lymphocyte count was not completely elucidated. We only demonstrated a few correlations between the dosimetric variables and some clinical variables, whereas dosimetric variables constitutionally interact with each other and with most clinical variables. In addition, our findings focus on the volume of low-dose radiation because we hypothesize it to be more clinically relevant, and future studies are needed to validate our findings.
In conclusion, our study showed that a higher NLR-b and NLR-h predicted adverse survival outcomes, despite the absence of any correlation between NLR and FFLR. NLR is thus a promising indicator of systemic inflammation and disease status, at different time points. Tumor size constitutes systemic inflammation and is related to NLR, and the target volume of low- dose radiation in both lungs and heart impact inflammation-immunity during treatment. RT dosage experienced by the lungs is correlated with NLR, and by the heart to the extent of decrease in lymphocyte counts. We suggest that both heart and lung doses should be optimized and minimized during radiation treatment, with an emphasis on low-dose volume. The use of advanced techniques, such as proton therapy, may serve as a fundamental strategy to reduce unnecessary exposure to radiation.
Acknowledgments
We would like to thank all members of the Department of Radiation Oncology, Division of Medical Physics, and Division of Hematology/Oncology and those who had collaborated to the patient treatments in this study. We also thank the editor and series editor for constructive criticisms of an earlier version of this chapter. This study was performed in accordance with ethical standards.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tro-20-62
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tro-20-62). JCL serves as an unpaid editorial board member of Therapeutic Radiology and Oncology from May 2020 to Apr 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Changhua Christian Hospital (CCH IRB No. 180310) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623-7. [Crossref] [PubMed]
- Nutting CM, Bedford JL, Cosgrove VP, et al. A comparison of conformal and intensity-modulated techniques for oesophageal radiotherapy. Radiother Oncol 2001;61:157-63. [Crossref] [PubMed]
- Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys 2010;76:S70-6. [Crossref] [PubMed]
- Asakura H, Hashimoto T, Zenda S, et al. Analysis of dose-volume histogram parameters for radiation pneumonitis after definitive concurrent chemoradiotherapy for esophageal cancer. Radiother Oncol 2010;95:240-4. [Crossref] [PubMed]
- Farr KP, Khalil AA, Knap MM, et al. Development of radiation pneumopathy and generalised radiological changes after radiotherapy are independent negative prognostic factors for survival in non-small cell lung cancer patients. Radiother Oncol 2013;107:382-8. [Crossref] [PubMed]
- Lin JB, Hung LC, Cheng CY, et al. Prognostic significance of lung radiation dose in patients with esophageal cancer treated with neoadjuvant chemoradiotherapy. Radiat Oncol 2019;14:85. [Crossref] [PubMed]
- Xu C, Guo L, Liao Z, et al. Heart and lung doses are independent predictors of overall survival in esophageal cancer after chemoradiotherapy. Clin Transl Radiat Oncol 2019;17:17-23. [Crossref] [PubMed]
- Hancock SL, Donaldson SS, Hoppe RT. Cardiac disease following treatment of Hodgkin's disease in children and adolescents. J Clin Oncol 1993;11:1208-15. [Crossref] [PubMed]
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987-98. [Crossref] [PubMed]
- Dess RT, Sun Y, Matuszak MM, et al. Cardiac events after radiation therapy: combined analysis of prospective multicenter trials for locally advanced non-small-cell lung cancer. J Clin Oncol 2017;35:1395-402. [Crossref] [PubMed]
- Wang K, Eblan MJ, Deal AM, et al. Cardiac toxicity after radiotherapy for stage III non-small-cell lung cancer: pooled analysis of dose-escalation trials delivering 70 to 90 Gy. J Clin Oncol 2017;35:1387-94. [Crossref] [PubMed]
- Contreras JA, Lin AJ, Weiner A, et al. Cardiac dose is associated with immunosuppression and poor survival in locally advanced non-small cell lung cancer. Radiother Oncol 2018;128:498-504. [Crossref] [PubMed]
- Speirs CK, DeWees TA, Rehman S, et al. Heart dose is an independent dosimetric predictor of overall survival in locally advanced non-small cell lung cancer. J Thorac Oncol 2017;12:293-301. [Crossref] [PubMed]
- Barcellos-Hoff MH, Park C, Wright EG. Radiation and the microenvironment - tumorigenesis and therapy. Nat Rev Cancer 2005;5:867-75. [Crossref] [PubMed]
- Demaria S, Formenti SC. Sensors of ionizing radiation effects on the immunological microenvironment of cancer. Int J Radiat Biol 2007;83:819-25. [Crossref] [PubMed]
- Jarosz-Biej M, Smolarczyk R, Cichoń T, et al. Tumor microenvironment as a "game changer" in cancer radiotherapy. Int J Mol Sci 2019;20:3212. [Crossref] [PubMed]
- Davuluri R, Jiang W, Fang P, et al. Lymphocyte nadir and esophageal cancer survival outcomes after chemoradiation therapy. Int J Radiat Oncol Biol Phys 2017;99:128-35. [Crossref] [PubMed]
- Xu C, Jin JY, Zhang M, et al. The impact of the effective dose to immune cells on lymphopenia and survival of esophageal cancer after chemoradiotherapy. Radiother Oncol 2020;146:180-6. [Crossref] [PubMed]
- Venkatesulu BP, Mallick S, Lin SH, et al. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol Hematol 2018;123:42-51. [Crossref] [PubMed]
- Hu J, Chen D, Wu S, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in middle thoracic esophageal squamous cell carcinoma patients undergoing radical esophagectomy. J Thorac Dis 2020;12:363-74. [Crossref] [PubMed]
- Li KJ, Xia XF, Su M, et al. Predictive value of lymphocyte-to-monocyte ratio (LMR) and neutrophil-to-lymphocyte ratio (NLR) in patients with oesophageal cancer undergoing concurrent chemoradiotherapy. BMC Cancer 2019;19:1004. [Crossref] [PubMed]
- Pirozzolo G, Gisbertz SS, Castoro C, et al. Neutrophil-to-lymphocyte ratio as prognostic marker in esophageal cancer: a systematic review and meta-analysis. J Thorac Dis 2019;11:3136-45. [Crossref] [PubMed]
- Schrek R. Qualitative and quantitative reactions of lymphocytes to x rays. Ann N Y Acad Sci 1961;95:839-48. [Crossref] [PubMed]
- Yovino S, Kleinberg L, Grossman SA, et al. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest 2013;31:140-4. [Crossref] [PubMed]
- Borst J, Ahrends T, Babala N, et al. CD4+ T cell help in cancer immunology and immunotherapy. Nat Rev Immunol 2018;18:635-47. [Crossref] [PubMed]
- van der Leun AM, Thommen DS, Schumacher TN. CD8+ T cell states in human cancer: insights from single-cell analysis. Nat Rev Cancer 2020;20:218-32. [Crossref] [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245-51. [Crossref] [PubMed]
- Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017;541:321-30. [Crossref] [PubMed]
- Schernberg A, Blanchard P, Chargari C, et al. Neutrophils, a candidate biomarker and target for radiation therapy? Acta Oncol 2017;56:1522-30. [Crossref] [PubMed]
- Luo HS, Xu HY, Du ZS, et al. Prognostic significance of baseline neutrophil count and lactate dehydrogenase level in patients with esophageal squamous cell cancer treated with radiotherapy. Front Oncol 2020;10:430. [Crossref] [PubMed]
- Ishibashi Y, Tsujimoto H, Yaguchi Y, et al. Prognostic significance of systemic inflammatory markers in esophageal cancer: Systematic review and meta-analysis. Ann Gastroenterol Surg 2019;4:56-63. [Crossref] [PubMed]
- Liu CL, Lee JJ, Liu TP, et al. Blood neutrophil-to-lymphocyte ratio correlates with tumor size in patients with differentiated thyroid cancer. J Surg Oncol 2013;107:493-7. [Crossref] [PubMed]
- Al Lawati Y, Cools-Lartigue J, Ramirez-GarciaLuna JL, et al. Dynamic alteration of neutrophil-to-lymphocyte ratio over treatment trajectory is associated with survival in esophageal adenocarcinoma. Ann Surg Oncol 2020;27:4413-9. [Crossref] [PubMed]
- Sherry AD, Newman NB, Anderson JL, et al. Systemic inflammatory dynamics during chemoradiotherapy predict response, relapse, metastasis, and survival in esophageal carcinoma. J Surg Oncol 2019; Epub ahead of print. [Crossref] [PubMed]
- McLaughlin M, Patin EC, Pedersen M, et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer 2020;20:203-17. [Crossref] [PubMed]
- Tang C, Liao Z, Gomez D, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys 2014;89:1084-91. [Crossref] [PubMed]
- Deng W, Xu C, Liu A, et al. The relationship of lymphocyte recovery and prognosis of esophageal cancer patients with severe radiation-induced lymphopenia after chemoradiation therapy. Radiother Oncol 2019;133:9-15. [Crossref] [PubMed]
- Stratton JA, Byfield PE, Byfield JE, et al. A comparison of the acute effects of radiation therapy, including or excluding the thymus, on the lymphocyte subpopulations of cancer patients. J Clin Invest 1975;56:88-97. [Crossref] [PubMed]
- Sellins KS, Cohen JJ. Gene induction by gamma-irradiation leads to DNA fragmentation in lymphocytes. J Immunol 1987;139:3199-206. [PubMed]
- Nakamura N, Kusunoki Y, Akiyama M. Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay. Radiat Res 1990;123:224-7. [Crossref] [PubMed]
- Ladbury CJ, Rusthoven CG, Camidge DR, et al. Impact of radiation dose to the host immune system on tumor control and survival for stage III non-small cell lung cancer treated with definitive radiation therapy. Int J Radiat Oncol Biol Phys 2019;105:346-55. [Crossref] [PubMed]
- Fang P, Shiraishi Y, Verma V, et al. Lymphocyte-sparing effect of proton therapy in patients with esophageal cancer treated with definitive chemoradiation. Int J Part Ther 2018;4:23-32. [Crossref] [PubMed]
- Routman DM, Garant A, Lester SC, et al. A comparison of grade 4 lymphopenia with proton versus photon radiation therapy for esophageal cancer. Adv Radiat Oncol 2019;4:63-9. [Crossref] [PubMed]
Cite this article as: Ho YC, Lai YC, Lin HY, Ko MH, Wang SH, Yang SJ, Lin PJ, Chou TW, Hung LC, Huang CC, Chang TH, Lin JC, Lin JB. The volume of low-dose thoracic irradiation influences systemic inflammation-immunity status after chemoradiation in esophageal cancer. Ther Radiol Oncol 2021;5:9.


