
Adjuvant radiotherapy for dermatofibrosarcoma protuberans: a case report
Introduction
Dermatofibrosarcoma protuberans (DFSP) is an uncommon cutaneous sarcoma of fibroblast origin (1) DFSP constitutes approximately 1% of all sarcomas and <0.1% of all malignancies (2,3). These tumors tend to grow slowly and rarely metastasize (4,5). DFSP generally presents an asymptomatic, firm, protuberant swollen nodule or plaque (4,5). The typical presentation of DFSP is a long history (ranging from months to several years) of a slowly growing indurated dermal plaque or nodule with subsequent nodules appearing at later stages (5,6). Clinically, these are firm to hard lesions that are fixed to the skin and vary in color from brown to reddish-blue or violaceous (5,6).
The standard treatment for localized primary or recurrent DFSP is radical and wide local excision for achieving clear margins (7). The local recurrence rates after surgery have been reported to be 10–60% depending on the nature of the surgery and margin status of DFSP (7-12). Mohs micrographic surgery (a technique of repeated surgery involving immediate microscopic examination of the tumor margin after excision until excision is complete) and wide surgical excision with 2–4 cm margins are recommended for DFSP (8,11,12). However, Mohs micrographic surgery is time-consuming and is not suitable for large tumors, whereas wide excision with resection margins ≥3 cm may cause higher reconstruction rates (13).
For patients with DFSP in whom tumors cannot attain negative margins after surgery, adjuvant radiotherapy (RT) may be considered to improve local control and prevent undesired cosmetic or functional outcomes (14). Herein, we describe a rare case of DFSP in the right shoulder of a 25-year-old woman treated with excision and adjuvant RT.
Brief history
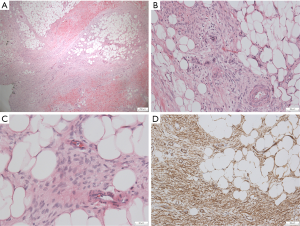
A 25-year-old woman had a 2×2 cm-sized red-purple painless erosion in the right shoulder about 10 years ago. The skin lesion changed to a painless plaque 1 year ago. However, the plaque enlarged gradually. She then underwent excisional biopsy at a local hospital in November 2015. Histological evaluation revealed an ill-defined lesion throughout the thickness of the dermis and largely extending to the subcutis, comprising slender to plump spindle cells with low to high cellularity, mild nuclear atypia, and various amounts of collagenous matrix. Mitoses were occasionally found. DFSP was diagnosed based on the morphologic findings and immunohistochemical staining, which showed positivity for cluster of differentiation 34 (CD34) and negativity for S-100. The maximum dimension of the tumor was 1.7 cm. The basal cut margins were involved by tumor. She visited the physician at our hospital. Under the suspicion of positive margins of postoperative DFSP, she underwent wide excision in December 2015. The pathology report showed some non-circumscribed hyper-cellular areas in scarring in the background, composed of thin spindle cells with scant cytoplasm, infiltrating subcutaneous tissue and entrapping adipose tissue (Figure 1). No conspicuous nuclear atypia or mitosis was observed. These tumor cells were diffusely positive for CD34 (Figure 1). In this case report, the expression of Ki-67 in tumor cells of this patient is around 3% to 5%. Foci of the section margins were close, but free. Because of the close section margins of DFSP, she then received adjuvant RT 1 month after re-excision. We administered intensity modulated radiation therapy (IMRT) to reduce the inhomogeneity and dose of the humeral head and improve the planned target volume dose coverage. The patient was immobilized with a prone pillow. A 0.5-cm bolus was placed on the skin to achieve adequate surface dose.
In this study, the gross tumor volume (GTV) [clinical target volume (CTV) boost] was defined as tumor bed (including surgical scar) according to post-operative MRI imaging and operation scar. To avoid anatomical boundaries (no penetrate deltoid muscle), the CTV was defined as GTV (CTV boost) plus 3 cm margins in the superior and inferior directions and 1 to 1.5 cm margins in the medial and lateral directions. The planning target volume (PTV) was defined as CTV plus 5 mm margin, and the PTV boost was defined as CTV boost with 5 mm margin. The prescribed radiation dose for this patient was 46 Gy with 2 Gy per fraction to the CTV and PTV followed by 14 Gy with 2 Gy per fraction to the CTV boost and PTV boost. We used four fields (Figure 2A), 6 MV, coplanar, IMRT plans that were generated on the Eclipse Treatment Planning System, Version 13.0. Considering the target volumes of this patient were shoulder and upper extremity, dose constraints for organ at risk (OAR) in this patient were as follows: V20 (volume receiving at least 20 Gy) for lung <10%, V30 (volume receiving at least 30 Gy) for bone <50% (included humerus), and maximal doses for esophagus and spinal cord were ≤15 Gy.
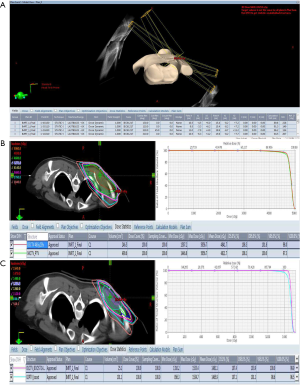
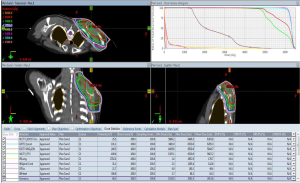
As shown in Figure 2B, the proportion of CTV receiving 95% and PTV receiving 95% of the prescription dose of 46 Gy were 98% and 97.3%, respectively. The proportion of CTV boost receiving 95% and PTV boost receiving 95% of the prescription dose of 14 Gy were 100% and 98.6%, respectively (Figure 2C). As shown in Figure 3, the humerus of V30 was less than 35%. The patient experienced the greatest acute toxicity of grade 2 skin reaction at irradiated field. Fortunately, she had neither local recurrence nor grade 2 to 3 late adverse effects following the irradiation during the 3-year follow-up. The study protocol of retrospective analyses of the relationship between adjuvant RT and clinical outcomes of sarcoma was approved by the Research Ethics Committee of National Taiwan University Hospital. The patients’ medical data were anonymized prior to the access and analysis.
Discussion
DFSP is a malignant fibroblastic tumor that most frequently arises in middle-aged individuals, with the peak age ranging from 25 to 45 years (4,5). The most common sites of DFSP are the trunk (40–50%), proximal extremities (30–40%), and head and neck (10–15%) (6,15). Morphologically, DFSP is characterized by a uniform population of small, slender to plump spindle cells with diffuse CD34 expression (6,15,16). However, the diagnosis for DFSP is frequently delayed because of many differential diagnoses (other spindle cell neoplasms) and the typically low clinical suspicion of malignancy in this slowly growing lesion (3,6,15). Despite frequent prolonged delays in the diagnosis of DFSP, metastasis to the lymph nodes or distant sites is very rare (<5%) (2,8); the most common site of metastasis for DFSP is the lungs (7).
DFSP consists of different histological subtypes, including conventional (classical) DFSP, sclerotic DFSP, giant cell fibroblastoma (a juvenile form of DFSP), pigmented DFSP (also known as Bednar tumor), myxoid DFSP, atrophic DFSP, and fibrosarcomatous DFSP (17,18). Among these subtypes, fibrosarcomatous DFSP is noticeable as the most aggressive subtype which has higher risks of local recurrences and distant metastases, whereas conventional DFSP is the most common subtypes and also has risk of local recurrence (around 20% to 50%) but rarely develops metastases (18-20). Pigmented DFSP, a rare neoplasm (around 5% of DFSP), presenting during early or middle adult life, is characterized by the histological manifestations of melanin-containing dendritic cells (most tumor cells are positive for CD34, like our case presented) within the tumor (21,22). Clinically, patients with pigmented DFSP have around 10% to 13% local recurrence rates and very rare distant metastases (21,22). Other subtypes of DFSP, such as sclerotic DFSP, giant cell fibroblastoma, myxoid DFSP, and atrophic DFSP were tremendously rare (17,18).
More than 90% of patients with DFSP harbor a specific reciprocal rearrangement of chromosomes 17 and 22: t(17;22)(q22;q13) (6,17,23). The rearrangement of t(17;22)(q22;q13) leads to the fusion of the platelet-derived growth factor beta (PDGFβ) gene with the collagen type 1 alpha 1 (COL1A1) gene (3,6,24). The formation of the COL1A1–PDGFβ fusion gene results in the constitutive upregulation in the expression of PDGFβ (24). PDGFβ has been reported to stimulate cell growth, differentiation, and migration of tumor cells in DFSP (25,26). Several reports have described the usefulness of imatinib, a tyrosine-kinase inhibitor that inhibits PDGF receptors, in the treatment of metastatic and localized unresectable DFSP (4,6).
Considering that Ki-67 is a protein that involved in cell proliferation and severed as a reliable marker for detecting proliferation activities of tumor cells (27), several studies reported the association of Ki-67 with clinicopathological manifestations of DFSP (28-30). Zorlu et al. reported that the Ki-67 labeling index was different in the primary tumor (2%), in the recurrent tumor (15% to 20%), and in metastatic sites of lung (70%) of a 22-year-old woman with a classical cutaneous DFSP (28). Gu et al. also described a case of congenital DFSP with fibrosarcomatous and myxoid change, in which the Ki-67 labeling index was higher in components of fibrosarcomatous area (11.8%) and of myxoid areas (19.8%), but was lower in ordinary (plaque-like) area (2.2%) (29). In a recent analyses of relationship between Ki-67 labeling index and prognosis of 56 cases of DFSP, Du et al. reported that the Ki-67 expression (≥17%) was associated with age (<50 years old) (P=0.047) and fibrosarcomatous histological subtype (P=0.003) (30). Du also showed that patients with high expression of Ki-67 in tumor cells had a poor 5-year disease-free survival (DFS) than those with low Ki-67 expression in tumor cells (35.8% vs. 87.8%, P=0.002) (30). These results indicated that higher expression of Ki-67 may be closely associated with aggressive histology subtype, such as fibrosarcomatous DFSP, and higher risk of recurrence and distant metastases of DFSP.
Because DFSP is a rare tumor, there are no prospective randomized trials evaluating adjuvant RT in decreasing local recurrence. Castle et al. retrospectively reviewed the outcomes of 53 patients with DFSP who were treated with preoperative RT (n=7; RT dose, 50–50.4 Gy) or postoperative RT (n=46; RT dose, 60–66 Gy) revealed an excellent local control rate of 93% after 10 years of implementing the combined modality approach (31). Williams et al. reported that 12 (92.3%) out of 13 patients with DFSP who underwent surgery followed by RT remained disease-free at a median follow-up of 10.5 years (32). Woo et al. also reported that the recurrence rate for patients with DFSP who underwent marginal excision was higher in the non-adjuvant RT group than that in the adjuvant RT group (60% vs. 0%) (13). A systemic review and meta-analysis of DFSP by Chen et al. demonstrated that surgery and adjuvant RT conferred a trend of lower recurrence rates than those conferred by surgery alone (odds ratio 0.31, P=0.07), in which the pooled estimate of the recurrence rate for the surgery and adjuvant RT group was 11.74% (33). Du et al. used propensity score-matched analysis to minimize the selection bias and found that DFS was significantly higher in the surgery and RT group than in the surgery group (5-year DFS, 88.1% vs. 56.2%, P=0.044) (30). These findings suggest that adjuvant RT following surgery is considered as a treatment modality for DFSP (Table 1). The risk of radiation-related toxicity for DFSP should be considered. For example, Sun et al. reported that 6 (28.6%) out of 11 patients who received adjuvant RT ranging from 46 to 68 Gy had grade 2 fibrosis or telangiectasia (34). Castle et al. showed that 7 patients (13%) had radiation-related complications at 5 and 10 years (31). Huber et al. reported 8 patients developed radiation-induced sarcoma, including DFSP, at their primary irradiated site after a median 7.35 years of RT (35). The prognosis of these patients was very poor, indicating that the possible cons of radiation-related sarcoma should be discussed with patients with DFSP who will receive adjuvant RT (35).
Table 1
| Authors, references | Case number and margin status | Radiation dose | Local control | Interpretation |
|---|---|---|---|---|
| Woo |
RT n=6 (negative margin n=2); |
60 Gy | 100% |
Adjuvant RT significantly reduced recurrence in the marginal excision cases |
| Du |
RT n=44; no RT n =140; |
50–66 Gy | 5-year DFS 88.1% |
DFS was higher in the OP + RT group |
| Williams |
n=13 (negative margin n=4 | 55.8–66 Gy | 92.3% (median follow-up 10.5 years) | RT may improve local control in patients with DFSP |
| Chen |
n=167 (positive/close margin n=92; meta-analysis) | 57–65 Gy | 88.26% | There was a trend that adjuvant RT had a lower recurrence rate than surgery alone (odds ratio 0.31, P=0.07) |
RT, radiotherapy; DFS, dermatofibrosarcoma protuberans; DFS, disease-free survival; OP, operation.
Conclusions
In summary, DFSP is a rare malignancy with a high recurrence rate, especially in patients with positive margin status. Adjuvant RT has the potential efficacy to reduce the local recurrence. Furthermore, for patients with large tumors, especially when wide excision with negative margins may cause undesired cosmetic or functional outcomes, postoperative RT is highly recommended. However, the dose-response relationship for adjuvant RT has not been established, and doses of 55–65 Gy have been used in most published studies, including our study. Recently, two studies evaluated the use of imatinib as a neoadjuvant therapy in decreasing the preoperative tumor size in DFSP (36,37). Further studies evaluating combined modalities including neoadjuvant imatinib treatment and reducing the need for wide resection margins followed by diminished RT fields to reduce late adverse effects in patients with DFSP, are warranted.
Acknowledgments
Funding: This study was supported by the following research grants: NTUH 108-S4143 from National Taiwan University Hospital.
Footnote
Conflicts of Interest: SHK serves as an Associate Editors-in-Chief of Therapeutic Radiology and Oncology from Oct 2017 to Sep 2019. MYH has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kohlmeyer J, Steimle-Grauer SA, Hein R. Cutaneous sarcomas. J Dtsch Dermatol Ges 2017;15:630-48. [PubMed]
- DuBay D, Cimmino V, Lowe L, et al. Low recurrence rate after surgery for dermatofibrosarcoma protuberans: A multidisciplinary approach from a single institution. Cancer 2004;100:1008-16. [Crossref] [PubMed]
- Llombart B, Serra-Guillén C, Monteagudo C, et al. Dermatofibrosarcoma protuberans: A comprehensive review and update on diagnosis and management. Semin Diagn Pathol 2013;30:13-28. [Crossref] [PubMed]
- Acosta AE, Vélez CS. Dermatofibrosarcoma Protuberans. Curr Treat Options Oncol 2017;18:56. [Crossref] [PubMed]
- Li Y, Wang C, Xiang B, et al. Clinical Features, Pathological Findings and Treatment of Recurrent Dermatofibrosarcoma Protuberans. J Cancer 2017;8:1319-23. [Crossref] [PubMed]
- Thway K, Noujaim J, Jones RL, et al. Dermatofibrosarcoma protuberans: pathology, genetics, and potential therapeutic strategies. Ann Diagn Pathol 2016;25:64-71. [Crossref] [PubMed]
- Rutgers EJ, Kroon BB, Albus-Lutter CE, et al. Dermatofibrosarcoma protuberans: Treatment and prognosis. Eur J Surg Oncol 1992;18:241-8. [PubMed]
- Farma JM, Ammori JB, Zager JS, et al. Dermatofibrosarcoma protuberans: How wide should we resect? Ann Surg Oncol 2010;17:2112-8. [Crossref] [PubMed]
- Gloster HM, Harris KR, Roenigk RK. A comparison between Mohs micrographic surgery and wide surgical excision for the treatment of dermatofibrosarcoma protuberans. J Am Acad Dermatol 1996;35:82-7. [PubMed]
- Monnier D, Vidal C, Martin L, et al. Dermatofibrosarcoma protuberans: a population-based cancer registry descriptive study of 66 consecutive cases diagnosed between 1982 and 2002. J Eur Acad Dermatol Venereol 2006;20:1237-42. [Crossref] [PubMed]
- Molina AS, Duprat Neto JP, Bertolli E, et al. Relapse in dermatofibrosarcoma protuberans: A histological and molecular analysis. J Surg Oncol 2018;117:845-50. [Crossref] [PubMed]
- Chang CK, Jacobs IA, Salti GI. Outcomes of surgery for dermatofibrosarcoma protuberans. Eur J Surg Oncol 2004;30:341-5. [Crossref] [PubMed]
- Woo KJ, Bang SI, Mun GH, et al. Long-term outcomes of surgical treatment for dermatofibrosarcoma protuberans according to width of gross resection margin. J Plast Reconstr Aesthet Surg 2016;69:395-401. [Crossref] [PubMed]
- Rutkowski P, Debiec-Rychter M. Current treatment options for dermatofibrosarcoma protuberans. Expert Rev Anticancer Ther 2015;15:901-9. [Crossref] [PubMed]
- Paramythiotis D, Stavrou G, Panagiotou D, et al. Dermatofibrosarcoma protuberans: a case report and review of the literature. Hippokratia 2016;20:80-3. [PubMed]
- John AM, Holahan HH, Singh P, et al. When Immunohistochemistry Deceives Us: The Pitfalls of CD34 and Factor XIIIa Stains in Dermatofibroma and Dermatofibrosarcoma Protuberans. Skinmed 2017;15:53-5. [PubMed]
- Socoliuc C, Zurac S, Andrei R, et al. A review of morphological aspects in dermatofibrosarcoma protuberans with clinicopathological correlations. Rom J Intern Med 2014;52:239-50. [PubMed]
- Kim GK. Status report on the management of dermatofibrosarcoma protuberans: is there a viable role for the use of imatinib mesylate? In which cases may it be therapeutically helpful and in which cases not? J Clin Aesthet Dermatol 2011;4:17-26. [PubMed]
- Voth H, Landsberg J, Hinz T, et al. Management of dermatofibrosarcoma protuberans with fibrosarcomatous transformation: an evidence-based review of the literature. J Eur Acad Dermatol Venereol 2011;25:1385-91. [Crossref] [PubMed]
- Ishida M, Okabe H. Fibrosarcomatous pigmented dermatofibrosarcoma protuberans: A case report with review of the literature. Oncol Lett 2012;4:390-2. [Crossref] [PubMed]
- Reis-Filho JS, Milanezi F, Ferro J, et al. Pediatric pigmented dermatofibrosarcoma protuberans (Bednár tumor): case report and review of the literature with emphasis on the differential diagnosis. Pathol Res Pract 2002;198:621-6. [Crossref] [PubMed]
- Suehara Y, Yazawa Y, Hitachi K. Metastatic Bednar tumor (pigmented dermatofibrosarcoma protuberans) with fibrosarcomatous change: a case report. J Orthop Sci 2004;9:662-5. [Crossref] [PubMed]
- Pedeutour F, Simon MP, Minoletti F, et al. Translocation, t(17;22)(q22;q13), in dermatofibrosarcoma protuberans: a new tumor-associated chromosome rearrangement. Cytogenet Cell Genet 1996;72:171-4. [Crossref] [PubMed]
- Llombart B, Sanmartín O, López-Guerrero JA, et al. Dermatofibrosarcoma protuberans: clinical, pathological, and genetic (COL1A1-PDGFB) study with therapeutic implications. Histopathology 2009;54:860-72. [Crossref] [PubMed]
- Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 1999;79:1283-316. [Crossref] [PubMed]
- Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev 2008;22:1276-312. [Crossref] [PubMed]
- Tsai YJ, Lin PY, Chew KY, et al. Dermatofibrosarcoma protuberans in children and adolescents: clinical presentation, histology, treatment, and review of the literature. J Plast Reconstr Aesthet Surg 2014;67:1222-9. [Crossref] [PubMed]
- Zorlu F, Yildiz F, Ertoy D, et al. Dermatofibrosarcoma Protuberans Metastasizing to Cavernous Sinuses and Lungs: a Case Report. Jpn J Clin Oncol 2001;31:557. [Crossref] [PubMed]
- Gu W, Ogose A, Kawashima H, et al. Congenital dermatofibrosarcoma protuberans with fibrosarcomatous and myxoid change. J Clin Pathol 2005;58:984. [Crossref] [PubMed]
- Du K, Li J, Tang L, et al. Role of postoperative radiotherapy in dermatofibrosarcoma protuberans: a propensity score-matched analysis. Radiat Oncol 2019;14:20. [Crossref] [PubMed]
- Castle KO, Guadagnolo BA, Tsai CJ, et al. Dermatofibrosarcoma protuberans: long-term outcomes of 53 patients treated with conservative surgery and radiation therapy. Int J Radiat Oncol Biol Phys 2013;86:585-90. [Crossref] [PubMed]
- Williams N, Morris CG, Kirwan JM, et al. Radiotherapy for dermatofibrosarcoma protuberans. Am J Clin Oncol 2014;37:430-2. [Crossref] [PubMed]
- Chen YT, Tu WT, Lee WR, et al. The efficacy of adjuvant radiotherapy in dermatofibrosarcoma protuberans: a systemic review and meta-analysis. J Eur Acad Dermatol Venereol 2016;30:1107-14. [Crossref] [PubMed]
- Sun LM, Wang CJ, Huang CC, et al. Dermatofibrosarcoma protuberans: treatment results of 35 cases. Radiother Oncol 2000;57:175-81. [Crossref] [PubMed]
- Huber GF, Matthews TW, Dort JC. Radiation-induced soft tissue sarcomas of the head and neck. J Otolaryngol 2007;36:93-7. [Crossref] [PubMed]
- Kérob D, Porcher R, Vérola O, et al. Imatinib mesylate as a preoperative therapy in dermatofibrosarcoma: results of a multicenter phase II study on 25 patients. Clin Cancer Res 2010;16:3288-95. [Crossref] [PubMed]
- Ugurel S, Mentzel T, Utikal J, et al. Neoadjuvant imatinib in advanced primary or locally recurrent dermatofibrosarcoma protuberans: a multicenter Phase II DeCOG trial with long-term follow-up. Clin Cancer Res 2014;20:499-510. [Crossref] [PubMed]
Cite this article as: Hu MY, Kuo SH. Adjuvant radiotherapy for dermatofibrosarcoma protuberans: a case report. Ther Radiol Oncol 2019;3:32.


