
Recurrent diffuse-type tenosynovial giant cell tumor of the left ankle: a case report
Introduction
“Tenosynovial giant cell tumor” refers to a group of diseases arising from the synovium of joint, bursa and tendon sheath. According to the site of origin (intra- or extra-articular) and the growth pattern (localized or diffuse), the disease can be divided into two main subtypes: localized type or diffuse type. These two subtypes have distinct clinical and biological features, but share common pathogenesis and pathological findings.
Diffuse-type tenosynovial giant cell tumor (D-TGCT), also called pigmented villonodular synovitis (PVNS), is one kind of rare, benign tumor. The incidence is around 1.8 per million people each year in the US. The incidence rate peaks in the third and fourth decades of life. There is slight female predominance. It is more locally aggressive and prone to recurrence compared to localized type. Compare to localized-type tenosynovial giant cell tumor (L-TGCT), D-TGCT has more infiltration and L-TGCT has more well circumscribes with at least partial covered by fibrous capsule. D-TGCT has more mitoses and more mitotic activity of >5 per 10 high power field (HPF) than L-TGCT of mitotic activity around 3–5 mitoses per 10 HPF.
Extensive synovectomy followed by low dose irradiation is the common therapy. Since D-TGCT is very rare, there may be delayed diagnosis and treatment. Moreover, there is no standardized treatment protocol. Here, we present a case of recurrent D-TGCT over left ankle receiving excision and adjuvant radiotherapy. The current literature regarding treatments for and prognosis of this disease was reviewed.
Case presentation
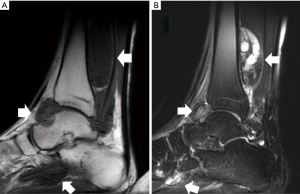
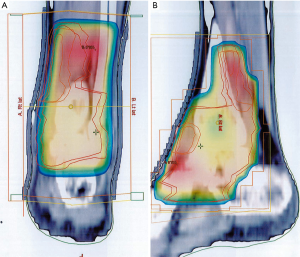
In March 2006, a 22-year-old male presented with progressive heat and swelling sensation over the left ankle. He denied trauma and relevant family history. Neither pain nor limited range of motion was noted. He had visited local Traditional Chinese Medicine clinics for 6 months. However, the discomfort progressed. Therefore, he came to our hospital and D-TGCT was suspected based on the results of magnetic resonance imaging (MRI) (Figure 1). He received open tumor marginal excision twice by a surgeon. The pathologic reports showed multiple fragments aggregating to 105×70×25 and 43×30×20 mm3, respectively, in greatest diameter of villus to lobular nodules. Under microscopy, there was a mixture of mononuclear and multinuclear histiocytes with a sprinkling of chronic inflammatory cells. There were also zonal arrangements of lipid and hemosiderin-laden histiocytes. Surgical margins could not be assessed due to the multiple small pieces. After the operation, there were no obvious complications. The discomfort including heat and swelling subsided and he could stand and walk normally. Adjuvant radiotherapy was delivered with 6 megavoltage (MV) photon beams by three-dimensional conformal radiation therapy (3D-CRT) technique once per day, with 5 fractions per week. The radiation fields included the initial tumor bed as clinical target volume (CTV). The CTV was expanded by 5mm to create planning target volume (PTV). The PTV include posterior tibia area, ankle joint, calcaneus talus joint, transverse tarsal joint, navicular cuneiform joints, navicular cuboidal joint and cuboidal cuneiform joint (Figure 2). The PTV received 35 gray (Gy) in 17 fractions.
After the treatment, the only adverse event from radiation was grade 1 dermatitis (CTCAE V3.0) which resolved a few weeks after finishing the radiation course.
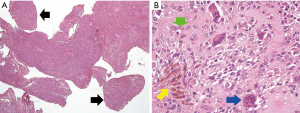
Nine years later, in October 2015, he complained of another episode of progressive heat and swelling sensation over the left ankle. Physical examination showed mild tenderness, swelling and disability without limited range of motion. MRI showed irregular soft tissue lesions along posterior distal tibia shaft extending to plantar area. Under the impression of recurrent D-TGCT with progression, open tumor marginal excision was carried out. The pathology report revealed giant cell tumor of tendon sheath with multiple fragments aggregating to 45×40×40 mm3 (Figure 3). A mixture of mononuclear and multinuclear histiocytes was found under microscopy. There was no adequate surgical margin and no necrotic tissue. After providing a thorough explanation to the patient, he received adjuvant radiotherapy again.
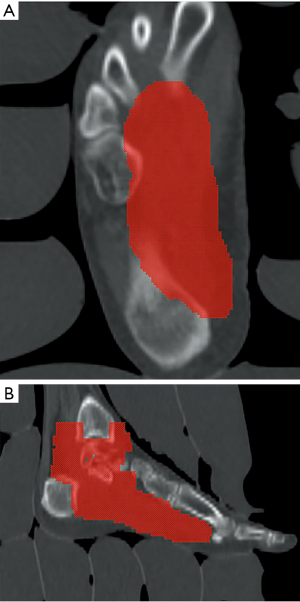
This time, radiotherapy was delivered with a 6 MV linear accelerator by photon beam using helical intensity modulated radiotherapy (IMRT) techniques (Accuray TomoHelical®), once per day, with 5 fractions per week. Normal saline bags were used as bolus to enhance the superficial tissue dose. The radiation fields included the tumor bed, ankle and plantar area as CTV. The CTV was expanded by 5 mm to create the PTV. The PTV includes plantar area from distal end of 1st, 2nd, 3rd and 4th metatarsal bone along the foot arch to plantar area of three cuneiform bone, cuboid bone and calcalcaneus. And it ascends to the calcaneus talus joint, fibular talus joint and tibia talus joint (Figure 4). The PTV received 48 Gy in 24 fractions. Treatment planning was carried out with Accuracy® HiArt® Software planning station (version 5). The PTV coverage data was 99% dose cover 99% volume.
During the radiotherapy period, acute treatment-related reactions included grade 1 radiation dermatitis according to CTCAE V3.0. The patient received regular follow-up after radiotherapy with complete physical examination at every visit and MRI every six months. The patient was followed up for 30 months after radiotherapy. There were no clinical symptoms but MRI still showed irregular soft tissue with suspicion of residual tumor. After radiotherapy twice with cumulative dose 83 Gy to the left ankle, poor skin condition of easy touch bleeding noted with grade 3 skin dermatitis, (CTCAE V5.0), easy swelling over the ankle joint after standing for a long time with grade 2 joint effusion (CTCAE V5.0) and the ankle joint decreased ROM with poor jump and run activity noted with grade 1 joint range of motion decreased (CTCAE V5.0). There were no other complications after the two radiation courses.
Discussion
Clinical features
D-TGCT usually involves single joint. The most commonly involved site is knee, followed by hip and ankle (80%, 15%, and 5% of cases, respectively). The symptoms vary depending on location and size of lesions. Sensation of warmth, swelling, pain, bony erosion, and, if located around a joint, limited range of motion may present. Under arthroscopy, there may be coarse villi, diffuse nodularity, heavy pigmentation (from dark yellow to chocolate brown), joint effusions and soft tissue masses.
D-TGCT is thought to be locally aggressive. It widely infiltrates and entraps adjacent soft tissues and frequently erodes the adjacent bone. As the clinical appearance is poorly defined, and the prevalence is rare, D-TGCT is easily misdiagnosed. In the largest report, which involved 237 patients, the median delay from initial clinical symptoms to final diagnosis was 18 months (1). Delayed management can lead to severe osteoarthritic deterioration with massive joint destruction which contraindicates conservative treatments. Due to high recurrence rates (18–46%) (2), the most extensive surgical resection possible should be performed, and adjuvant therapies are recommended.
For the past few decades, there has been a debate about the etiology of D-TGCT. It was described as an inflammatory process or benign neoplasm formation in 1941, when Jaffe et al. addressed PVNS as an inflammatory disease of synovial tissue caused by uncertain trauma (3). Recently, studies in the field of cytogenetics have alluded to the involvement of “tumor landscaping”. Neoplastic cells constitute a minor component within the tumor and overexpress colony stimulating factor 1 (CSF1) to recruit and activate nonneoplastic, inflammatory cells. New drugs have been developed to block the associated pathway.
Diagnosis
Early diagnosis is the first step in treating this rare disease to achieve good results. The average duration from symptoms to diagnosis has been reported to be between 10 and 26 months (4). For our patient, the duration from symptoms to diagnosis was only 6 months. Radiologically, D-TGCT usually presents as soft tissue mass with pressure erosion of nearby bone tissue from hyperplastic synovial growth inside a strong capsule inducing increased intraarticular pressure. Osteopenia can also be found in the juxtaarticular bone.
MRI is thought to be the best imaging method for diagnosis. Imaging studies can demonstrate synovial proliferation, joint effusion, underlying bone erosion and hemosiderin deposition within synovial masses (5). Lesions are usually described as hypointense on T1-weighted images and heterogeneous with decreased signal intensity on T2-weighted images. This is due to hemosiderin content (6). In this case, heterogeneous long T2 image with intra-articular and extra-articular involvement and underlying bone erosion and low signal image on T1-weighted image with intra-articular and extra-articular involvement were noted.
Fludeoxyglucose positron emission tomography-computed tomography (FDG PET-CT) is not routinely used for diagnosis, as images can mimic other benign tumors. Broski et al. reported 14 cases of D-TGCT receiving FDG PET-CT with a mean standardized uptake value (SUV) max of 8.7 (4.0–14.5 g/mL). They concluded that D-TGCT is intensively hyper metabolic in PET and mimics musculoskeletal metastasis. Once D-TGCT has been diagnosed, it may be a useful tool for monitoring treatment response (7).
Definitive diagnosis can only be made by pathologic examination. In 1941, Jaffe et al. first created the term “PVNS” for the histological appearance of this disease characterized by lipid laden macrophages, multinucleated giant cells, and deposits of hemosiderin within a fibrous stroma (8). In 2002, World Health Organization proposed a new definition of destructive proliferation of synovial-like mononuclear cells, mixed with multinucleated giant cells, foam cells, siderophages and inflammatory cells (9). Mononuclear cells express clusterin and a small portion stain for desmin in most cases. Multinucleate giant cells show osteoclastic phenotype and express CD68, CD45. Due to typical morphology under microscopy with mixture of mononuclear and multinuclear histiocytes, these specimens did not undergo further immunohistochemistry (IHC) staining. Some reports have shown that highly cellular tumors with increased mitotic activity and diffuse forms are associated with high recurrence rates (10).
Treatment strategies
Surgical removal
Generally, D-TGCT is treated with surgical removal of all affected tissues. The operation often requires a relatively wide incision and a large detachment of the soft tissues due to the high recurrence rate related to incomplete resection. Large incision carries a risk of infection, necrosis and mechanically alterable scar. All bone lesions should be treated with careful curettage followed by bone graft if necessary. Among ankle lesions, there is frequently extra-articular involvement. Open synovectomy for ankle cases is the first line treatment, allowing for complete excision of lesions. In our case, this patient received 3 open tumor marginal excisions. For shoulder and knee joint lesions, arthroscopic surgery provides direct visualization and easy accessibility. It also decreases the major complications of joint stiffness by small and nonproblematic scars, postoperative pain, and rehabilitation time. For extensive lesions or extraarticular involvement, arthroscopic and open surgery should be combined to avoid failure. Compared with lesions located at knee, lesions at ankle have higher recurrence rate (0–40% vs. 4.7–16.1%) due to more extra-articular involvement (11).
Adjuvant external beam radiotherapy
Due to high recurrence rate, many studies have suggested adjuvant treatment. In 1935, Kling and Sashin first introduced partial synovectomy followed by radiotherapy as an alternative to total synovectomy which may have fewer complications (12). Berger et al. reported 7 cases: 5 knee, 1 hip, 1 wrist, post radical operation with residual disease. Four were primary disease cases. Three were recurrent disease cases with repeated surgical interventions. After receiving a mean dose of 40 Gy in 20 fractions, no patient had evidence of recurrent or persistent disease after 29-month follow-up. It has been suggested that 40–45 Gy in 20 fractions is adequate (13). Heyd et al. retrospectively reviewed 41 cases receiving post-operative radiotherapy in 19 institutions in Germany from 1990–2008. Thirty were residual tumors, 11 were free from margin. Total dose ranged from 30–54 Gy with median dose of 36 Gy over median fraction size of 2 Gy in five weekly fractions. After follow-up periods from 6 months to more than 10 years, two in-field recurrences (4.9%) were observed. The remaining 39 patients (95.1%) achieved local control. Two recurrent cases received a total dose of 36.0 Gy, applied in single doses of 1.8 Gy in one case and 2.0 Gy in the other case. Minor side effects were noted. Acute toxicities or late sequelae greater than Radiation Oncology Treatment Group (RTOG) grade II were not observed. It has been demonstrated that radiotherapy achieves a high local rate in the postoperative setting after nonradical resection and as a salvage treatment option for recurrent and refractory disease (14). Blanco et al. presented 22 patients with D-TGCT of the knee who underwent adjuvant radiotherapy with a total dose of 26 Gy followed by partial (anterior) arthroscopic synovectomy. Only 3 (14%) cases had confirmed relapse treated with salvage arthroscopic surgery (15).
Adjuvant radiosynoviorthesis
Intra-articular injection of radionuclide (radiosynoviorthesis) has also been considered as an adjuvant therapy. Emission radionuclides such as yttrium-90 (Y-90) or dysprosium-165 (Dy-165) may be potent enough to eliminate residual D-TGCT. Koca et al. reported 15 cases with knee joint involvement who underwent post-operative radiosynovectomy with 5 millicurie (mCi) Y-90. After median follow up for 50 (range, 12–86) months, no tumor recurrence was found and symptoms significantly improved based on Lysholm knee score (16). Ottaviani et al. reviewed 122 cases and suggested Y-90 for lesions over the knee joints (4–5 mCi) and rhenium-186 for lesions over the hip (3 mCi) and ankle joints (2 mCi). During treatment, isotopic injection is under radioscopic control via iodine intra-articular injection. Isotopic synoviorthesis should not be performed if bone lesions are important or prosthetic replacement is considered. In children, isotopic synoviorthesis is also contraindicated (17). However, there is some skepticism that extra-articular or extensive DPVNS can be controlled sufficiently using Y-90 or Dy-165 with maximal synovial penetrations of 11 and 5.7 mm, respectively (16).
Drugs
Recent advances in cytogenetics have revealed that D-TGCT is the result of translocation (1;2)(p13;q37) causing fusion of CSF1-COL6A3 genes (18) and overexpression of CSF1. CSF1 is also known as macrophage CSF (M-CSF). It is a secreted cytokine that plays an essential role in proliferation, differentiation, and survival of monocytes, macrophages, and related cells. However, neoplastic cells constitute a minor component within the tumor, accounting for only 2% to 16% of cells. Most cells are nonneoplastic, inflammatory cells which are recruited and activated by CSF1 produced by the neoplastic cells. This phenomenon is called “tumor landscaping”. This crucial role of aberrant CSF1 signaling in the development and progression of D-TGCT makes this pathway an ideal therapeutic target for recurrent or unresectable disease. It has the potential to be the first-line treatment for diffuse lesions to prevent perioperative morbidities arising from total synovectomy or total joint replacement. Cassier et al. recruited 29 D-TGCT patients treated with emactuzumab, a recombinant humanized monoclonal antibody of IgG1, directly against CSF1R expressed on macrophage. One patient had stable disease, two patients had complete response and others had partial response. All but one was clinically disease free. Based on European Organization for Research and Treatment of Cancer (EORTC) criteria, most adverse events were grade 1 or 2. Asthenia (56%), pruritus (56%), facial edema, including periorbital, eyelid edema (64%), and peripheral edema (36%) were frequently reported adverse events (19). Blay et al. reported a case who received imatinib, a CSF1R blockade, with complete remission in the 5th month and stoppage of the drug in the 7th month. Painful events with disease relapse were noted in the 9th month. After reintroducing the drug, a second complete remission was observed in the 12th month (20). Table 1 shows the results of previous studies on D-TGCT.
Table 1
| Study group | Case No. | Location | Treatment | Follow up (months) | Local control (%) |
|---|---|---|---|---|---|
| Zhao et al. [2016] (10) | 1 | Ankle | Tumor incision | 12 | 100 |
| Ottaviani et al. [2011] (17) | 50 | Knee | Surgical synovectomy + isotopic synoviorthesis | 55 | 70 |
| Ottaviani et al. [2011] (17) | 23 | Other than knee | Surgical synovectomy + isotopic synoviorthesis | 40 | 91 |
| Koca et al. [2013] (16) | 15 | Knee | Surgical synovectomy + isotopic synoviorthesis | 48 | 100 |
| Berger et al. [2007] (13) | 7 | 5 knee, 1 hip, 1 wrist | Radical synovectomy + EBRT | 29 | 100 |
| Heyd et al. [2010] (14) | 41 | 25 knee, 8 ankle, 8 others | Surgical intervention + EBRT | 63 | 95 |
| Blanco et al. [2001] (15) | 22 | Knee | Surgical intervention + EBRT | 33 | 86 |
| Wu et al. [2019] | 1 | Ankle | Surgical intervention + EBRT | 30 | 100 |
| Cassier et al. [2015] (19) | 29 | 15 knee, 8 foot or ankle, 6 others | Emactuzumab | 12 | 100 |
EBRT, external beam radiotherapy.
Here we present a refractory case of D-TGCT over left ankle who received marginal excision followed by adjuvant radiotherapy. There was relapse 9 years later and he received operation and adjuvant radiotherapy again. This is the first case to be reported in which radiotherapy was administered twice with relatively tolerable late side effects. In recurrent cases, adjuvant radiotherapy is still an effective adjuvant treatment. Regular follow-up is needed. If disease recurs, one of the recently developed drugs may also be considered.
Acknowledgments
Cheryl Robbins, a professional English editor, helps language editing assistance.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (Available at https://tro.amegroups.com/article/view/10.21037/tro-03-17/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xie GP, Jiang N, Liang CX, et al. Pigmented villonodular synovitis: a retrospective multicenter study of 237 cases. PLoS One 2015;10:e0121451. [Crossref] [PubMed]
- Myers BW, Masi AT, Feigenbaum SL. Pigmented villonodular synovitis and tenosynovitis: a clinical epidemiologic study of 166 cases and literature review. Medicine 1980;59:223-38. [Crossref] [PubMed]
- Jaffe HL, Lichtenstein L, Sutro CJ. Pigmented villonodular synovitis, bursitis and tenosynovitis. A discussion of synovial and bursal equivalents of the tenosynovial lesion commonly denoted as xanthorna, xanthogranuloma, giant cell tumor or myeloplaxoma of the tendon sheath, with some consideration of this tendon sheath lesion itself. Arch Pathol 1941;31:731-65.
- Bouali H, Deppert EJ, Leventhal LJ, et al. Pigmented villonodular synovitis: a disease in evolution. J Rheumatol 2004;31:1659-62. [PubMed]
- Cheng XG, You YH, Liu W, et al. MRI features of pigmented villonodular synovitis (PVNS). Clin Rheumatol 2004;23:31-4. [Crossref] [PubMed]
- Jelinek JS, Kransdorf M, Shmookler B, et al. Giant cell tumor of the tendon sheath: MR findings in nine cases. AJR Am J Roentgenol 1994;162:919-22. [Crossref] [PubMed]
- Broski SM, Murdoch NM, Skinner JA, et al. Pigmented villonodular synovitis: potential pitfall on oncologic 18F-FDG PET/CT. Clin Nucl Med 2016;41:e24-31. [Crossref] [PubMed]
- Gao M, Li H, Liang X, et al. Multifocal pigmented villonodular synovitis coexisting in both the knee joint and the patella: a case report and literature review. BMC Musculoskelet Disord 2017;18:293. [Crossref] [PubMed]
- Fletcher CD, Unni KK, Mertens F. Pathology and genetics of tumours of soft tissue and bone. IARC, 2002.
- Zhao JJ, Xie M, Huang RK, et al. Diffuse type tenosynovial giant cell tumor of the ankle. Chin Med J (Engl) 2016;129:881-2. [Crossref] [PubMed]
- Noailles T, Brulefert K, Briand S, et al. Giant cell tumor of tendon sheath: Open surgery or arthroscopic synovectomy? A systematic review of the literature. Orthop Traumatol Surg Res 2017;103:809-14. [Crossref] [PubMed]
- Kling DH, Sashin D. Hemorrhagic villous synovitis of the knee joint due to xanthoma: report of a case. Arch Surg 1935;30:52-61. [Crossref]
- Berger B, Ganswindt U, Bamberg M, et al. External beam radiotherapy as postoperative treatment of diffuse pigmented villonodular synovitis. Int J Radiat Oncol Biol Phys 2007;67:1130-4. [Crossref] [PubMed]
- Heyd R, Micke O, Berger B, et al. Radiation therapy for treatment of pigmented villonodular synovitis: results of a national patterns of care study. Int J Radiat Oncol Biol Phys 2010;78:199-204. [Crossref] [PubMed]
- Blanco CER, Leon HO, Guthrie TB. Combined partial arthroscopic synovectomy and radiation therapy for diffuse pigmented villonodular synovitis of the knee. Arthroscopy 2001;17:527-31. [Crossref] [PubMed]
- Koca G, Ozsoy H, Atilgan HI, et al. A low recurrence rate is possible with a combination of surgery and radiosynovectomy for diffuse pigmented villonodular synovitis of the knee. Clin Nucl Med 2013;38:608-15. [Crossref] [PubMed]
- Ottaviani S, Ayral X, Dougados M, et al. Pigmented villonodular synovitis: a retrospective single-center study of 122 cases and review of the literature. Semin Arthritis Rheum 2011;40:539-46. [Crossref] [PubMed]
- Mondal K, Mandal R, Khan K, et al. Pitfalls in the cytological diagnosis of tenosynovial giant cell tumor: An illustration of eight discordant cases. Diagn Cytopathol 2018;46:250-7. [Crossref] [PubMed]
- Cassier PA, Italiano A, Gomez-Roca CA, et al. CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: a dose-escalation and dose-expansion phase 1 study. Lancet Oncol 2015;16:949-56. [Crossref] [PubMed]
- Blay JY, El Sayadi H, Thiesse P, et al. Complete response to imatinib in relapsing pigmented villonodular synovitis/tenosynovial giant cell tumor (PVNS/TGCT). Ann Oncol 2008;19:821-2. [Crossref] [PubMed]
Cite this article as: Wu CC, Chou YH, Yang WJ, Hsu JD, Tseng HC, Lee YC. Recurrent diffuse-type tenosynovial giant cell tumor of the left ankle: a case report. Ther Radiol Oncol 2019;3:17.


