
Comparing treatment plans for proximal and middle/distal stomach cancer: intensity-modulated radiotherapy, volumetric-modulated arc therapy, and tomotherapy
Introduction
Stomach cancer (SC) is one of the most common causes of death from cancer worldwide (1). The disease is pervasive worldwide, especially in Asia, and is often diagnosed in advanced stages. According to the stage stratified by clinical stage groupings, and based on data in the National Cancer Database, less than 40% of patients are alive 5 years after a diagnosis of locally advanced gastric cancer with regional lymph node (LN) metastasis. However, since the successful intervention in eradication of Helicobacter pylori and the widespread availability of food refrigeration, there has been a significant downward trend in the overall incidence of gastric cancer over the past 50 years. Moreover, progress in cancer treatment including modern radiotherapy (RT) and chemotherapy provide more treatment options for SC. Regarding the decrease in the rates of locoregional and distant recurrence following surgery, a study showed that multimodality therapies, such as adjuvant chemoradiation therapy, benefit patients with gastric cancer in intermediate stages (2). A randomized phase III trial, Intergroup 0116 (INT-0116), was conducted to compare observation versus postoperative chemoradiotherapy following R0 resection of the gastroesophageal (GE) junction and gastric adenocarcinoma (3). The landmark INT-0116 trial and the updated 10-year follow-up revealed that there was a marked benefit from adjuvant chemotherapy and RT for curatively resected gastric cancer and GE junction cancer, especially in T3 or greater primaries and positive LNs. Besides the diminishing incidence of gastric cancer, a notable tumor distribution was observed. That is, a significant anatomic shift of gastric tumor has been recognized in recent years, whereby the rate of non-cardia tumors has declined while the incidence of cardia tumors has increased (4). Blaser et al. indicated an 11-fold increase in proportion of proximal SC from 0.4% to 4.5% in past five decades in Japan (5).
Although advances in RT have occurred over decades, the application of contemporary RT techniques as an adjuvant-based treatment for gastric cancer has not been clarified. Regarding concern for surrounding healthy tissue, a comparison on the normal tissue sparing of modern planning techniques with regard to different tumor locations of SC should be elucidated. Serarslan et al. has reported IMRT appears to achieve superior OAR protection than either wedge-based conformal RT or field-in-field IMRT in antrum-located SC (6). Current highly conformal treatment modalities, including intensity-modulated radiation therapy (IMRT), volumetric-modulated arc therapy (VMAT), and helical tomotherapy (TOMO) should be analyzed carefully with respect to the dose distributions in the target volume and organs at risk (OARs) based on different anatomical subsites for gastric cancer.
Accordingly, the aims of this study were to compare modern RT planning techniques on the basis of similar target coverage and to determine which technique fits a particular location of gastric cancer more in respect to the dose distribution to planning target volume (PTV) and OARs, namely the heart, lungs, bilateral kidneys, liver, small bowel, and spinal cord.
Methods
Patient data
We enrolled 32 newly diagnosed patients with gastric cancer receiving adjuvant RT after gastrectomy from January 2013 to May 2017. Our study is approved by local IRB and our IRB number is TMU-JIRB No.:N210712030. The first eligible criterion was pathology-proven gastric adenocarcinoma in patients that had received a total gastrectomy. The location of the primary lesion was recorded. Overall, 11 gastric cancer patients with six proximal and five middle/distal lesions were evaluated. A patient selection flowchart is presented in Figure 1. All anatomical locations of stomach adenocarcinoma were included. Other eligibility criteria were patients aged 20 to 90 years who had a favorable Eastern Cooperative Oncology Group (ECOG) performance status (score 0, 1, or 2). Tumors were staged according to the seventh edition of the American Joint Committee on Cancer (AJCC) staging system [2010] and were categorized as pathological T3–T4b and positive nodes. Because the PTV varied considerably owing to the location of the primary tumor and extent of surgical resection, the primary tumor bed and regional LNs were the target of radiation as per a consensus approved by the Multidisciplinary Gastrointestinal Tumor Board at Shuang Ho Hospital. The patient characteristics were summarized in Table 1.
Table 1
| Patient characteristic | Groups | Proximal (n=6), n (%) | Middle/Distal (n=5), n (%) |
|---|---|---|---|
| Gender | Female | 0 (0) | 1 (20.0) |
| Male | 6 (100.0) | 4 (80.0) | |
| Age (years) | <65 | 4 (66.7) | 4 (80.0) |
| ≥65 | 2 (33.3) | 1 (20.0) | |
| Median (range) | 61.0 (48.0–87.0) | ||
| ECOG | 0 | 3 (50.0) | 3 (60.0) |
| 1 | 2 (33.3) | 1 (20.0) | |
| 2 | 1 (16.7) | 1 (20.0) | |
| BMI | Underweight (≤18.5) | 2 (33.3) | 0 (0) |
| Normal weight (18.5–24.9) | 4 (66.7) | 5 (100.0) | |
| Overweight (25–29.9) | 0 (0) | 0 (0) | |
| Obesity (BMI ≥30) | 0 (0) | 0 (0) | |
| GERD | − | 5 (83.3) | 2 (40.0) |
| + | 1 (16.7) | 3 (60.0) | |
| Tobacco use | − | 3 (50.0) | 3 (60.0) |
| + | 3 (50.0) | 2 (40.0) | |
| Alcoholic drinking | − | 5 (83.3) | 3 (60.0) |
| + | 1 (16.7) | 2 (40.0) | |
|
|
− | 6 (100.0) | 4 (80.0) |
| + | 0 (0) | 1 (20.0) | |
| Previous stomach surgery | − | 6 (100.0) | 5 (100.0) |
| + | 0 (0) | 0 (0) | |
| Pernicious anemia | − | 6 (100.0) | 5 (100.0) |
| + | 0 (0) | 0 (0) | |
| Surgery | Total gastrectomy | 6 (100.0) | 5 (100.0) |
| Subtotal gastrectomy | 0 (0) | 0 (0) | |
| LN dissection | D1 | 3 (50.0) | 1 (20.0) |
| D2 | 3 (50.0) | 4 (80.0) | |
| AJCC stage | IIIA | 2 (33.3) | 1 (20.0) |
| IIIB | 1 (16.7) | 2 (40.0) | |
| IIIC | 2 (33.3) | 1 (20.0) | |
| IV | 1 (16.7) | 1 (20.0) | |
P/M/D, proximal/ middle/distal; Tx, treatment.
Simulation and target delineation
All patients were immobilized in a vacuum bag in the supine position with their arms placed above their head. A computed tomography (CT) scan of the chest and abdomen was completed after at least 4 hours of fasting and oral contrast was used to identify intestinal loops. Respiration control and abdominal compressor were not used. We performed RT planning on a contrast-enhanced CT image with an interval of 5 mm in slide thickness. The target volumes and OARs were contoured according to the International Commission on Radiation Units and Measurements (reports 50 and 62) and all delineations were performed on individual axial CT slices (7). The clinical target volume (CTV) comprised the original tumor bed and regional draining LSNs at risk, depending on the tumor location. The PTV was defined as a 5–10-mm expansion to the CTV to account for daily setup error and organ motion. OARs comprised the heart, lungs, bilateral kidneys, liver, small bowel, and spinal cord. The small bowel was delineated an additional 1 cm in both the superior and inferior edge of the PTV.
Planning requirement and techniques
The treatment plans were generated using IMRT, VMAT, and TOMO techniques. Irrespective of whether or not the patients had an initial prospective IMRT, VMAT, or TOMO plan, all treatments were re-planned retrospectively to enable a comparison of optimized IMRT, VMAT, and TOMO dosimetric parameters. All RT plans were completed by the same medical physicist. A total radiation dose of 50.4 Gy in 28 fractions was prescribed as adjuvant RT for gastric cancer, which is required to obtain a similar PTV coverage in all plans. The objective of planning was to deliver 97% of the prescription dose (D97) to cover at least 97% of the PTV. All treatment plans aimed to achieve a minimum dose higher than 97% and a maximum dose lower than 115% of the prescribed dose. The dose constraints for IMRT, VMAT, and TOMO plans are listed in Table 2 (8).
Table 2
| OARs | Prescribed dose limit |
|---|---|
| Heart | Dmean <26 Gy |
| V30 <46% | |
| V25 <10% | |
| Lung | V20 <30% |
| Spinal cord | Dmax <50 Gy |
| Whole liver | Dmean <30 Gy |
| Bilateral whole kidneys | Dmean <18 Gy |
| V20 <32% | |
| V23 <30% | |
| V28 <20% | |
| Small bowel | V45 <195 c.c. |
OARs, organs at risk; Vx, percentage of volume receiving at least x Gy.
IMRT and VMAT planning with a 10-MV photon beam were performed on the Pinnacle treatment planning system (version 9.8.0; Philips, Fitchburg, WI, USA) and irradiation was delivered using a linear accelerator equipped with multileaf collimators. The IMRT plans comprised six coplanar beams. We accomplished IMRT plans by trial and error and angles of 20°, 80°, 170°, 220°, 300° and 340° for proximal gastric cancer and angles of 30°, 80°, 100°, 140°, 280° and 340° for middle/distal gastric cancer were found to achieve the provided constraints. VMAT plans comprised double arcs with a collimator angle of 10°, rotating clockwise from 181° to 180° and then 180° to 181° counterclockwise. A maximum delivery time of 200 seconds/arc was used during the optimization.
A 6-MV photon energy beam with a helical fan-beam was used in TOMO. TOMO plans with a field width of 2.5 cm, pitch of 0.287, and modulation factor of 3.5 were generated using a TOMO planning system (Hi-Art Tomotherapy 4.1.2; TomoTherapy Inc., Madison, WI, USA).
CT contours and OARs were drawn in Pinnacle version 9.8 and transmitted to the TOMO planning system. Treatment plans were assessed on the basis of similar PTV coverage and were compared with each technique in relation to the conformity and homogeneity, as well as the dose distribution in OARs. The conformity index (CI) and homogeneity index (HI) were calculated as follows:
VT,ref is the volume of the target covered by the reference isodose line, VT is the PTV, and Vref is the volume covered by the reference isodose line. A value closer to 1 implies better conformity of the dose to the PTV (9).
DX represents the minimum dose to the x% of the PTV exposed to the highest dose and Dpre is the prescribed dose. D2 represents the average maximum dose for the PTV, whereas D98 represents the average minimum dose for the PTV. A lower HI suggests superior uniformity of the distributed dose (6).
Statistical analyses
Statistical analyses was conducted using Statistical Package for Social Sciences (SPSS) 19 (IBM SPSS Inc., Armonk, NY) and data were demonstrated as the mean and standard deviation. The differences in dosimetric parameters among three planning techniques, and two groups, each with a different tumor location, were evaluated using Wilcoxon’s signed-rank test: IMRT vs. VMAT, IMRT vs. TOMO, and VMAT vs. TOMO. Dosimetric results of various planning techniques and comparison of organs at risk in the proximal and middle/distal location tumor were assessed using independent paired t-test. A P value <0.05 was regarded as statistically significant.
Results
Patient characteristics
Patient data of the 11 participants in this study were collected retrospectively from January 2013 to May 2017 and comprised six patients with proximal gastric cancer and five with middle/distal gastric cancer after receiving total gastrectomy combined with adjuvant chemotherapy and RT in Shuang Ho Hospital. There was only one female patient and the median age of these patients was 61 years (ranging from 48 to 87 years). Regarding the ECOG performance status, more than half of the patients had a score of zero. Regarding body mass index, none of the patients were overweight at the diagnosis. Nine patients had stage III cancer, which included three stage IIIA, three stage IIIB, and three stage IIIC based on the seventh edition of the AJCC. Two patients were stage IV with solitary liver metastases and were treated on a salvage basis. All patients received total gastrectomy with regional LN dissection. Seven patients (63%) underwent D2 dissection (LN ≥15). No difference was observed between the proximal and middle/distal groups with respect to age at diagnosis, sex, ECOG performance status, stage, and surgical procedure.
Target volume doses
TOMO, VMAT, and IMRT all achieved desirable PTV coverage, with 97% of the prescribed dose (D97) covering at least 97% of the PTV. No significant difference was observed in hot spot doses and HI among the three techniques. However, TOMO seemed to have a superior CI to IMRT in patients with proximal gastric cancer (P=0.033). In terms of the monitor units (MUs), there were fewer MUs (shorter treatment time) in IMRT and VMAT than in TOMO. The dosimetric parameters for target volumes are summarized in Table 3.
Table 3
| Variable | Mean ± SD | P value | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IMRT | VMAT | TOMO | IMRT |
IMRT |
VMAT |
||||||||||||
| Proximal | Middle/Distal | Proximal | Middle/Distal | Proximal | Middle/Distal | P | M/D | P | M/D | P | M/D | ||||||
| D97 (PTV48.88) (%) | 97.24±0.27 | 97.19±0.18 | 97.23±0.30 | 97.28±0.20 | 98.26±0.94 | 97.99±1.14 | 1.000 | 1.000 | 0.200 | 0.563 | 0.237 | 0.802 | |||||
| CTV48.88 (%) | 99.90±0.17 | 99.95±0.12 | 99.88±0.11 | 99.93±0.15 | 99.47±1.17 | 99.82±0.41 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | |||||
| Hot spot (Gy) | 5,444.75±138.81 | 5,504.40±117.78 | 5,519.97±100.92 | 5,602.04±142.66 | 5,452.00±104.30 | 5,539.60±91.99 | 0.464 | 0.144 | 1.000 | 0.967 | 1.000 | 0.362 | |||||
| HI | 0.07±0.04 | 0.10±0.03 | 0.10±0.02 | 0.12±0.03 | 0.08±0.04 | 0.10±0.03 | 0.578 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | |||||
| CI | 0.82±0.03 | 0.83±0.02 | 0.80±0.05 | 0.82±0.04 | 0.85±0.02 | 0.85±0.02 | 0.571 | 1.000 | 0.033* | 0.879 | 0.062 | 0.657 | |||||
| MU (sec in Tomo) | 462.43±82.69 | 418.94±116.12 | 468.25±61.01 | 447.80±112.06 | 6,432.70±571.74 | 5,337.74±556.70 | 1.000 | 1.000 | 0.028* | 0.043* | 0.028* | 0.043* | |||||
| Right kidney | |||||||||||||||||
| Mean dose (cGy) | 989.40±461.79 | 974.76±668.93 | 1,002.50±419.16 | 853.12±608.77 | 845.83±415.80 | 828.80±470.45 | 1.000 | 1.000 | 0.153 | 0.978 | 0.297 | 1.000 | |||||
| V20 (%) | 17.14±9.49 | 14.20±14.68 | 16.03±9.40 | 10.75±11.18 | 9.46±8.03 | 6.29±8.34 | 1.000 | 0.810 | 0.112 | 0.551 | 0.264 | 0.402 | |||||
| V23 (%) | 11.55±6.88 | 10.00±10.25 | 13.21±8.60 | 7.32±8.55 | 6.23±5.60 | 3.08±4.36 | 1.000 | 0.726 | 0.023* | 0.354 | 0.207 | 0.345 | |||||
| V28 (%) | 7.01±5.99 | 4.31±4.62 | 8.91±6.75 | 3.63±5.29 | 3.27±3.38 | 0.22±0.37 | 1.000 | 1.000 | 0.071 | 0.333 | 0.214 | 0.652 | |||||
| Left kidney | |||||||||||||||||
| Mean dose (cGy) | 1,499.63±276.12 | 1,407.20±433.64 | 1,431.77±232.25 | 1,334.18±488.85 | 754.33±580.50 | 1,145.80±584.26 | 0.429 | 1.000 | 0.081 | 0.224 | 0.147 | 0.240 | |||||
| V20 (%) | 25.13±6.34 | 22.73±6.82 | 22.69±5.00 | 19.46±10.34 | 13.50±5.95 | 15.28±11.02 | 0.483 | 0.804 | 0.001* | 0.223 | <0.001* | 0.090 | |||||
| V23 (%) | 20.23±5.01 | 16.70±6.84 | 18.78±4.34 | 15.43±8.90 | 10.48±4.33 | 12.53±9.24 | 0.632 | 1.000 | 0.003* | 0.538 | <0.001* | 0.095 | |||||
| V28 (%) | 13.69±3.74 | 10.50±5.57 | 13.47±4.05 | 10.98±7.63 | 6.76±3.49 | 8.66±6.78 | 1.000 | 1.000 | 0.001* | 1.000 | 0.006* | 0.143 | |||||
| Total kidney | |||||||||||||||||
| Mean dose (cGy) | 1,255.52±251.90 | 1,225.28±572.06 | 1,229.88±254.07 | 1,123.18±547.99 | 1,002.00±269.10 | 1,014.00±545.75 | 0.429 | 0.146 | <0.001* | 0.010* | 0.005* | 0.049* | |||||
| V20 (%) | 21.33±5.57 | 19.26±10.70 | 19.64±4.72 | 15.73±10.45 | 11.77±5.51 | 11.20±9.00 | 0.227 | 0.030* | 0.009* | 0.011* | 0.013* | 0.014* | |||||
| V23 (%) | 15.98±3.49 | 14.19±8.73 | 16.23±4.20 | 12.01±8.55 | 8.39±4.04 | 8.34±6.51 | 1.000 | 0.020* | 0.001* | 0.014* | 0.018* | 0.063 | |||||
| V28 (%) | 10.41±3.39 | 8.25±5.47 | 11.35±3.69 | 7.96±6.32 | 5.05±2.95 | 5.14±4.39 | 1.000 | 1.000 | <0.001* | 0.253 | 0.027* | 0.356 | |||||
| Liver | |||||||||||||||||
| Mean dose (cGy) | 1,664.62±127.73 | 1,763.56±291.20 | 1,722.27±93.53 | 1,908.54±591.61 | 1,555.00±158.24 | 1,813.80±666.64 | 0.600 | 1.000 | 0.320 | 1.000 | 0.122 | 1.000 | |||||
| V20 (%) | 31.15±4.58 | 34.38±5.70 | 32.10±2.62 | 41.93±23.42 | 22.71±6.96 | 40.35±22.12 | 0.917 | 0.893 | 0.028* | 0.686 | 0.046* | 0.500 | |||||
| V30 (%) | 16.66±1.25 | 18.99±8.22 | 18.24±2.08 | 20.05±11.30 | 9.93±3.74 | 18.42±11.57 | 0.420 | 1.000 | 0.009* | 1.000 | 0.019* | 0.751 | |||||
| Heart | |||||||||||||||||
| Mean dose (cGy) | 1,683.68±737.78 | 506.40±340.66 | 1,584.02±671.27 | 581.80±429.44 | 1,264.00±555.86 | 440.40±272.76 | 0.227 | 1.000 | 0.083 | 1.000 | 0.095 | 1.000 | |||||
| D1/3 (cGy) | 2,060.52±997.99 | 440.75±422.14 | 1917.69±907.49 | 470.18±363.10 | 1,389.50±661.63 | 359.12±207.61 | 1.000 | 1.000 | 0.046* | 0.178 | 0.046* | 0.043* | |||||
| V30 (%) | 17.72±12.23 | 3.38±3.30 | 15.72±10.23 | 4.34±4.80 | 8.61±5.93 | 2.11±2.38 | 0.477 | 1.000 | 0.082 | 0.234 | 0.064 | 0.583 | |||||
| Spinal cord Dmax (cGy) | 4,225.68±589.38 | 3,472.46±1,142.82 | 4,222.62±555.26 | 3,849.66±736.40 | 4,069.17±225.78 | 3,672.20±836.94 | 0.917 | 0.138 | 0.345 | 0.686 | 0.249 | 0.893 | |||||
| Small bowel | |||||||||||||||||
| Mean dose (cGy) | 26.07±6.69 | 27.84±9.68 | 27.94±5.96 | 29.69±7.40 | 26.62±7.46 | 28.28±8.37 | 0.046* | 0.686 | 0.753 | 0.500 | 0.345 | 0.500 | |||||
| V35 (c.c.) | 64.75±75.42 | 79.6220±67.60 | 69.80±78.65 | 78.92±64.31 | 54.82±67.55 | 72.06±59.13 | 0.046* | 0.893 | 0.075 | 0.225 | 0.075 | 0.225 | |||||
| V40 (c.c.) | 42.72±53.98 | 64.47±58.80 | 47.78±58.62 | 64.90±56.29 | 32.10±42.04 | 56.11±47.13 | 0.293 | 0.686 | 0.028* | 0.345 | 0.028* | 0.345 | |||||
| V45 (c.c.) | 29.80±30.92 | 39.13±52.15 | 34.99±34.68 | 39.29±52.76 | 22.16±22.76 | 29.71±38.44 | 0.669 | 1.000 | 0.474 | 1.000 | 0.361 | 1.000 | |||||
| Whole lung | |||||||||||||||||
| Mean dose (cGy) | 545.60±307.07 | 246.08±131.93 | 576.50±354.70 | 259.40±133.89 | 590.83±336.43 | 274.20±133.07 | 0.638 | 0.311 | 0.267 | 0.136 | 1.000 | 0.876 | |||||
| V5 (%) | 27.83±14.79 | 13.15±7.34 | 29.77±16.67 | 14.11±7.72 | 29.80±17.33 | 13.01±8.70 | 0.259 | 0.063 | 0.477 | 1.000 | 1.000 | 1.000 | |||||
| V10 (%) | 18.26±10.90 | 7.46±4.14 | 19.79±12.87 | 7.76±4.20 | 20.33±14.20 | 7.19±5.47 | 0.422 | 1.000 | 0.691 | 1.000 | 1.000 | 1.000 | |||||
| V20 (%) | 8.08±5.26 | 2.67±1.45 | 8.79±6.88 | 2.81±1.38 | 8.32±6.78 | 4.57±5.15 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | |||||
| V30 (%) | 3.96±3.32 | 0.96±0.72 | 4.20±4.22 | 1.04±0.80 | 3.71±3.27 | 1.15±0.94 | 1.000 | 0.459 | 1.000 | 1.000 | 1.000 | 1.000 | |||||
*, quick reference guide is based on the significant P value (P<0.05); otherwise, data without * means statistical insignificance. IMRT, intensity modulated radiation therapy; VMAT, volumetric modulated radiation therapy; TOMO, tomotherapy; SD, standard deviation; Heart D1/3, the mean dose covering one-third volume of the heart; VX, the percentage of organ receiving more or equal to x Gy; Dmax, maximum dose of certain OAR.
OAR doses
IMRT vs. VMAT
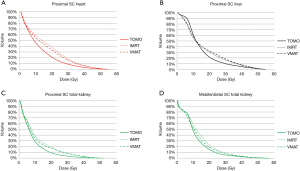
In the six patients diagnosed with proximal gastric cancer, IMRT lowered small bowel mean dose and V35 (P=0.046 and P=0.046, respectively) compared to VMAT. For middle/ distal tumors, VMAT lowered the dose in the bilateral kidney V20 and V30 (P=0.030 and P=0.020, respectively) compared with IMRT. The dose-volume histogram (DVH) results were shown in Figure 2.
IMRT vs. TOMO
In comparison with IMRT, TOMO lowered the heart D1/3 (P=0.046); left kidney V20 (P=0.001), V23 (P=0.003), and V28 (P=0.001); total kidney Dmean (P<0.001), V20 (P=0.009), V23 (P=0.001), and V28 (P<0.001); right kidney V23 (P=0.023); liver V20 (P=0.028) and V30 (P=0.009) and small bowel V40 (P=0.028) in patients with proximal gastric cancer. For patients with middle/distal gastric cancer, there was significantly lower total kidney Dmean (P=0.010), V20 (P=0.011), and V23 (P=0.014) when planning with TOMO rather than IMRT. The dose-volume histogram (DVH) results were shown in Figure 2.
VMAT vs. TOMO
For proximal gastric cancer, the TOMO treatment plan lowered the heart D1/3 (P=0.046); left kidney V20 (P<0.001), V23 (P<0.001), and V28 (P=0.006); total kidney Dmean (P=0.005), V20 (P=0.013), V23 (P=0.018), and V28 (P=0.027); liver V20 (P=0.046) and V30 (P=0.019); and small bowel V40 (P=0.028) compared with VMAT. In patients with middle/distal gastric tumor, TOMO significantly improved dose sparing to total kidney Dmean (P=0.049) and V20 (P=0.014). The dose-volume histogram (DVH) results were shown in Figure 2.
Proximal vs. middle/distal
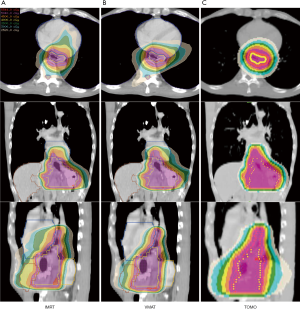
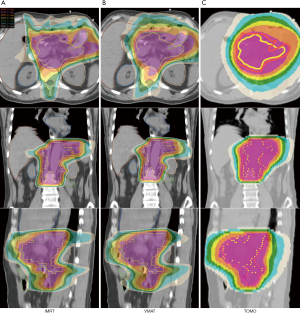
When comparing the dosimetric results between patients with proximal and middle/distal gastric cancer, TOMO significantly lowered the treatment time (MU) in the patients with middle/distal gastric cancer. In comparison with the heart dose in patients with proximal and middle/distal gastric cancer, all heart parameters, including heart Dmean, heart D1/3 and V30 were significantly higher in proximal gastric tumors than in the distal counterparts when planning with IMRT and VMAT. Moreover, the lung dose tended to be lower in middle/distal gastric tumors than in proximal tumors. Table 4 presents the dosimetric results for the planning parameters and a comparison for OARs in the proximal versus middle/distal tumor locations. Figures 3 and 4 show representative cases of proximal and middle/distal gastric cancer respectively.
Table 4
| Variable | Proximal |
||
|---|---|---|---|
| IMRT | VMAT | TOMO | |
| D97 (PTV48.88) (%) | 0.716 | 0.780 | 0.680 |
| CTV48.88 (%) | 0.614 | 0.471 | 0.542 |
| Hot spot (Gy) | 0.468 | 0.293 | 0.178 |
| HI | 0.132 | 0.363 | 0.351 |
| CI | 0.389 | 0.433 | 0.728 |
| MU | 0.486 | 0.708 | 0.011* |
| Right kidney | |||
| Mean dose (cGy) | 0.967 | 0.642 | 0.951 |
| V20 (%) | 0.696 | 0.416 | 0.538 |
| V23 (%) | 0.772 | 0.286 | 0.334 |
| V28 (%) | 0.433 | 0.190 | 0.078 |
| Left kidney | |||
| Mean dose (cGy) | 0.677 | 0.673 | 0.296 |
| V20 (%) | 0.561 | 0.513 | 0.740 |
| V23 (%) | 0.348 | 0.434 | 0.666 |
| V28 (%) | 0.287 | 0.505 | 0.562 |
| Total kidney | |||
| Mean dose (cGy) | 0.917 | 0.704 | 0.966 |
| V20 (%) | 0.688 | 0.473 | 0.901 |
| V23 (%) | 0.684 | 0.312 | 0.987 |
| V28 (%) | 0.443 | 0.295 | 0.970 |
| Liver | |||
| Mean dose (cGy) | 0.469 | 0.462 | 0.377 |
| V30 (%) | 0.563 | 0.707 | 0.122 |
| V20 (%) | 0.750 | 0.058 | 0.116 |
| Heart | |||
| Mean dose (cGy) | 0.010* | 0.018* | 0.015* |
| D1/3 (cGy) | 0.008* | 0.009* | 0.009* |
| V30 (%) | 0.032* | 0.049* | 0.048* |
| Spinal cord Dmax (cGy) | 0.105 | 0.238 | 0.010* |
| Small bowel | |||
| Mean dose (cGy) | 0.934 | 0.251 | 0.163 |
| V35 (%) | 0.432 | 0.669 | 0.173 |
| V40 (%) | 0.530 | 0.731 | 0.276 |
| V45 (%) | 0.720 | 0.874 | 0.712 |
| Whole lung | |||
| Mean dose (cGy) | 0.074 | 0.085 | 0.074 |
| V5 (%) | 0.067 | 0.078 | 0.073 |
| V10 (%) | 0.062 | 0.073 | 0.077 |
| V20 (%) | 0.053 | 0.088 | 0.338 |
| V30 (%) | 0.080 | 0.128 | 0.116 |
†, by independent
Discussion
Our study presented a dosimetric comparison of SC in different locations. As mentioned previously, adjuvant RT has played a crucial role in the postoperative setting for resectable gastric cancer since the landmark INT-0116 trial. However, the radiation technique traditionally employed in INT-0116 is considered to be outdated. Consequently, in this study, radiation was delivered using highly conformal radiation techniques such as IMRT, VMAT, and TOMO to attain extensive normal tissue sparing. Furthermore, we examined whether there was a dosimetric advantage in applying a particular planning technique to gastric tumors in different locations, which has never been discussed in the literature, and which drew our attention due to a marked anatomic shift in gastric cancer. That is, the incidence of gastric cardia tumors has increased while the incidence of distal gastric tumors has decreased (4). We endeavored to identify the optimal RT techniques for treating gastric cancer in shared or similar locations. In our study, patients diagnosed with gastric cancer who received a total gastrectomy were categorized into proximal (cardia) and middle/distal (body and antrum) groups.
Studies have demonstrated that IMRT has an edge over 3D conformal RT in terms of dosimetric parameters and doses of OARs (6,10-12). However, few studies have investigated comparisons of IMRT, VMAT, and TOMO in treating postoperative gastric cancer patients. We examined three contemporary planning techniques (IMRT, VMAT, and TOMO) that are commonly used in RT in Taiwan. As for the amounts of the beam angle design in IMRT, Nazareth et al. pointed the beam-angle selection which involves the selection of 5–10 angles from 360 gantry angles is crucial in IMRT planning (13). And Jia et al. further added that the beam orientation selection is usually achieved via a cumbersome trial-and-error approach conducted by experienced treatment planners (14). On the basis of same defined goal of the PTV coverage and OARs constraints, our 6-beam IMRT achieved the goal as VMAT did. We did not add more beams not only for the long and intolerable treatment time but also for the evidence that adding more beams in static IMRT increases the MUs and number of segments without any considerable improvement in dose distribution, leading to more leakage radiation and increased critical organ dose (15). We obtained satisfactory D97 covering at least 97% of the PTV, which is higher than the coverage reported in the study of Serarslan et al. (6), which discussed the treatment of antrum-located SC using IMRT and CRT. Moreover, our HI is comparable to theirs. We also obtained a similar CI as the data shown in the studies of Onal et al. (6,16), and a higher CI than that presented in Serarslan et al. (6).
No marked difference was noted in the locations of the tumors in our study and we had balanced cases in the proximal and middle/distal groups. We revealed that TOMO provided superior dose sparing for heart, total kidney, left kidney and liver V20 and liver V30 in proximal SC compared with IMRT and VMAT. By contrast, the benefit of TOMO planning for middle/distal SC was only observed in the total kidney dose. Moreover, a comparison of different locations was conducted on the basis of the same planning techniques. Heart dose was significantly higher in proximal gastric tumor than in the distal counterparts when planning with all three techniques, which is reasonably contributes to the closer distance to the heart.
The kidneys are vital organs, which are at risk in gastric cancer RT owing to the radiosensitivity of renal tissue. Cassady studied clinical radiation nephropathy and found that a total kidney dose of 18–23 Gy was associated with a 5% risk of injury within 5 years. Further investigation analyzing kidney function in SC patients treated with RT found that the mean renal dose was less crucial than V20, with the author recommending that <70% of the ipsilateral kidney volume receive 20 Gy (V20 <70%) (17). In our study, the total kidney constraint was set at V20 <32%. Our results of the kidney mean dose were compatible with another TOMO study, whereby Dahele et al. (18,19) reported mean right and left kidney doses of 13.6 Gy (range, 7.9–28.8 Gy) and 17.6 Gy (range, 14.9–19.4 Gy), respectively, which are higher than our results: Right kidneymean =8.4 Gy (range, 4.2–12.6 Gy) and Left kidneymean =9.3 Gy (range, 3.4–15.2 Gy). In proximal gastric cancer, TOMO significantly spared the left kidney and total kidney doses.
Radiation-induced liver disease has been a critical issue for patients with primary liver cancer treated with radiation. Because of the large radiation volume required for adjuvant RT of SC and the fact that the liver consumes a considerable proportion of the space in the upper abdomen, it is crucial to protect the liver from radiation injury when using adjuvant chemoradiation therapy to treat gastric cancer (20). In our study, TOMO planning was superior to both IMRT and VMAT in liver V20 and liver V30 (the percentage of the organ receiving more or equal to 30 Gy) in the proximal gastric cancer subgroup. According to an investigation by Gu et al. (11), liver V30–V40 is correlated with liver function injury. Therefore, we extrapolate that TOMO could reduce damage to liver function in patients with proximal gastric cancer receiving adjuvant RT.
In terms of the OAR constraints, other than the kidney and liver doses, we usually pay more attention to the dose to the heart and lungs when we encounter proximal SC because of their anatomic locations. Similarly, the cardiac exposure of radiation in treating breast cancer has been a pertinent. A population-based case-control study of the risk of ischemic heart disease in women receiving postoperative RT conducted by Darby et al. (18) showed a linear correlation between the mean heart dose and major coronary events, which increased by 7.4% per Gy. Additionally, cardiac radiation exposure increases the risk of heart failure with preserved ejection fraction in older women (mean age: 69±9 years) after breast irradiation (odds ratio: 16.9), with the odds being even higher than for heart failure with reduced ejection fraction (21). Correspondingly, the stomach is located on the left side of body as is the heart; therefore, the cardiac radiation dose administered in adjuvant RT of gastric cancer is of importance. In this study, we revealed that all parameters of heart dose (Dmean, D1/3 and V30) in proximal SC were significantly higher than that for middle/distal SC when comparing the heart dose of proximal and middle/distal gastric cancer. When comparing different planning techniques, we observed that TOMO treatment plans surpass either IMRT or VMAT plans on the heart dose in proximal SC. That is, TOMO yields a lower cardiac radiation dose in proximal SC. The highlight of our results indicated that TOMO achieved the most favorable dose improvements in terms of heart sparing in proximal gastric cancer.
A major limitation of this study is that the small sample size might have been too small to show a significant analytical difference between the groups. Other than advanced image-guided RT, we should consider more motion management strategies by using 4D CT, respiratory gating, or an abdominal compressor to reduce the shift of target volumes that are susceptible to substantial respiratory movement. It is believed that tumor motion can compromise the effectiveness and accuracy of radiation treatment. Clinically, either respiration motion or changes in the filling status of gastrointestinal organs causes tumor motion in the abdomen. Hu et al. (22) showed combining breath hold with image guided IMRT can minimize the organ motion and make the setup more accurate. Based on their dosimetric comparison, the dose could be escalated to 54 Gy without increasing the critical organs toxicities. With the addition of contemporary conformal techniques and the introduction of image-guided RT, closer target volume margins could be achieved without compromising the PTV coverage in addition to more favorable normal tissue sparing.
In summary, this is the first study to compare conformal RT techniques using IMRT, VMAT, and TOMO with regard to the PTV and OARs in adjuvant RT for gastric cancer in different locations. The results revealed that in the adjuvant treatment of gastric cancer, TOMO not only provided superior dose sparing for total kidney, liver V20, and liver V30, which was especially evident in proximal gastric cancer, but also provided the optimal dose improvements in the heart among the three planning techniques in proximal SC. Further study is required to validate the clinical use of TOMO in RT treatment plans for patients with gastric cancer.
Acknowledgments
This study was assisted by Department of Radiation Oncology, Shuang Ho Hospital. This manuscript was edited by Wallace Academic Editing.
Funding: This paper is funded by Shuang-Ho Hospital (grant number: 108SHHR-01).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Our study is approved by local IRB and our IRB number is TMU-JIRB No.:N210712030. Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Sandler S. Esophagogastric junction and gastric adenocarcinoma: neoadjuvant and adjuvant therapy, and future directions. Oncology (Williston Park) 2014;28:505-12. [PubMed]
- Smalley SR, Benedetti JK, Haller DG, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol 2012;30:2327-33. [Crossref] [PubMed]
- Camargo MC, Anderson WF, King JB, et al. Divergent trends for gastric cancer incidence by anatomical subsite in US adults. Gut 2011;60:1644-9. [Crossref] [PubMed]
- Blaser MJ, Saito D. Trends in reported adenocarcinomas of the oesophagus and gastric cardia in Japan. Eur J Gastroenterol Hepatol 2002;14:107-13. [Crossref] [PubMed]
- Serarslan A, Ozbek Okumus N, Gursel B, et al. Dosimetric Comparison of Three Different Radiotherapy Techniques in Antrum-Located Stomach Cancer. Asian Pac J Cancer Prev 2017;18:741-6. [PubMed]
- Purdy JA. Current ICRU definitions of volumes: limitations and future directions. Semin Radiat Oncol 2004;14:27-40. [Crossref] [PubMed]
- Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys 2010;76:S10-19. [Crossref] [PubMed]
- Wu VW, Kwong DL, Sham JS. Target dose conformity in 3-dimensional conformal radiotherapy and intensity modulated radiotherapy. Radiother Oncol 2004;71:201-6. [Crossref] [PubMed]
- Minn AY, Hsu A, La T, et al. Comparison of intensity-modulated radiotherapy and 3-dimensional conformal radiotherapy as adjuvant therapy for gastric cancer. Cancer 2010;116:3943-52. [Crossref] [PubMed]
- Gu L, Han Y, Li Y, et al. Emergence of Lamivudine-Resistant HBV during Antiretroviral Therapy Including Lamivudine for Patients Coinfected with HIV and HBV in China. PLoS One 2015;10:e0134539 [Crossref] [PubMed]
- Liu GF, Bair RJ, Bair E, et al. Clinical outcomes for gastric cancer following adjuvant chemoradiation utilizing intensity modulated versus three-dimensional conformal radiotherapy. PLoS One 2014;9:e82642 [Crossref] [PubMed]
- Nazareth DP, Brunner S, Jones MD, et al. Optimization of beam angles for intensity modulated radiation therapy treatment planning using genetic algorithm on a distributed computing platform. J Med Phys 2009;34:129-32. [Crossref] [PubMed]
- Jia X, Men C, Lou Y, et al. Beam orientation optimization for intensity modulated radiation therapy using adaptive l(2,1)-minimization. Phys Med Biol 2011;56:6205-22. [Crossref] [PubMed]
- Popple RA, Fiveash JB, Brezovich IA. Effect of beam number on organ-at-risk sparing in dynamic multileaf collimator delivery of intensity modulated radiation therapy. Med Phys 2007;34:3752-9. [Crossref] [PubMed]
- Onal C, Dolek Y, Akkus Yildirim B. Dosimetric comparison of 3-dimensional conformal radiotherapy, volumetric modulated arc therapy, and helical tomotherapy for postoperative gastric cancer patients. Jpn J Radiol 2018;36:30-9. [Crossref] [PubMed]
- Cassady JR. Clinical radiation nephropathy. Int J Radiat Oncol Biol Phys 1995;31:1249-56. [Crossref] [PubMed]
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987-98. [Crossref] [PubMed]
- Dahele M, Skinner M, Schultz B, et al. Adjuvant radiotherapy for gastric cancer: A dosimetric comparison of 3-dimensional conformal radiotherapy, tomotherapy and conventional intensity modulated radiotherapy treatment plans. Med Dosim 2010;35:115-21. [Crossref] [PubMed]
- Cheng JC, Liu HS, Wu JK, et al. Inclusion of biological factors in parallel-architecture normal-tissue complication probability model for radiation-induced liver disease. Int J Radiat Oncol Biol Phys 2005;62:1150-6. [Crossref] [PubMed]
- Saiki H, Petersen IA, Scott CG, et al. Risk of Heart Failure With Preserved Ejection Fraction in Older Women After Contemporary Radiotherapy for Breast Cancer. Circulation 2017;135:1388-96. [Crossref] [PubMed]
- Hu W, Ye J, Wang J, et al. Incorporating breath holding and image guidance in the adjuvant gastric cancer radiotherapy: a dosimetric study. Radiat Oncol 2012;7:98. [Crossref] [PubMed]
Cite this article as: Chen YC, Lin JC, Liu WH, Huang SF, Chou YC, Li MH, Tsai JT. Comparing treatment plans for proximal and middle/distal stomach cancer: intensity-modulated radiotherapy, volumetric-modulated arc therapy, and tomotherapy. Ther Radiol Oncol 2019;3:4.



