
A brief review on reactor-based neutron sources for boron neutron capture therapy
Introduction
All boron neutron capture therapy (BNCT) activity, research and clinic, has been done, until few years ago, using fission reactor neutron sources. Usually existing research reactors has been adapted to extract neutron beams useful for boron concentration measurement, for in vitro and in vivo preclinical experiments and for patient irradiation in suitable treatment rooms built in the reactor hall. There are two exceptions: the Massachusetts Institute of Technology Research Reactor (MITR) and the Brookhaven Medical Research Reactor (BMRR) commissioned in 1950s (1); more recently, in 2010, an in-hospital neutron irradiator (IHNI) was realized in Beijing, China (2,3) and one is in project in Nakhon Ratchasima, Thailand (Sanguansak N, 2018, unpublished data), both based on miniature reactor neutron source (MRNS).
At present only few reactor based BNCT sources are still active (Argentine, China, Japan, Taiwan) (4-7) and many accelerator-based neutron capture therapy (NCT) sources are already built or under installation. Although new accelerator-based sources will surely help BNCT diffusion, nonetheless what played by nuclear reactors was essential for BNCT and the current available reactors will continue to give a fundamental contribution to the further development of BNCT.
In this article, we report a brief review of the fission reactor-based neutron sources for BNCT [High Flux Reactor at Petten in the Netherlands, Studsvik reactor in Sweden, FiR1 reactor in Finland, LVR-15 reactor in Rez, Czech Republic, Kyoto University Research Reactor in Japan, JRR4 at JAER1 in Japan, Musashi Institute of Technology Reactor (KURR) in Japan, McCellan Nuclear Radiation Center Reactor at Davis, California, Washington State University reactor in Pullman, Washington, RA-6 reactor in Bariloche, Argentina, Tsing-Hua Open-pool Reactor (THOR) in Taiwan, Tapiro reactor in Rome, Italy, Triga Mark II of Pavia University in Italy] (8-26). We try to make a list of the different kind of sources and focus on the main requirements which these sources are able to fulfil for BNCT application, not pretending to be exhaustive due to the very broad topic.
General requirements for reactor-based BNCT neutron sources
The majority of reactor sources, realized by modification of existing reactors, use neutrons coming directly from the core (after appropriate moderation and filtration) (27); but there is an example, at MITR-II, that uses a fast neutron converter (FCB) installed in a large thermal neutron beam (28,29).
The first BNCT facilities were realized in the early 1950’s and 1960’s at Brookhaven National Laboratory (BNL) (29), the first nuclear reactor designed specifically for medical research and therapy and at MIT (MITR-I) with thermal neutron beams (30).
After the unsuccessful results of these trials (30) due to some reasons, among which the low capability of thermal neutrons to penetrate deeply into tissue, research on epithermal neutron beams for BNCT started. Figure 1 shows the different thermal neutron profile in tissue when a beam of thermal or epithermal neutrons is used. Calculations have been done assuming an ideal parallel neutron beam with different energy (0.0253 eV, 50 eV, 1 keV and 10 keV) impinging on a phantom with a standard soft tissue composition. It is evident that the penetrability in tissues of epithermal neutrons with the possibility to reduce the dose at patient surface are better than that of thermal neutrons; the maximum of thermal flux is reached at a depth of around 2–3 cm with neutron energies from 50 eV to 10 keV.
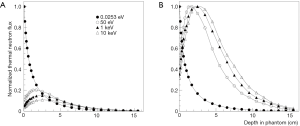
To be able to treat a patient in a reasonable time (no more than a few tenth of minutes for each irradiation field), taking into account a feasible level of boron concentration of about 30 ppm in the tumor, and to have a high probability to destroy the tumor, a thermal neutron fluence on the order on 1012 cm−2 is necessary. This means that a neutron flux on the order of 109 cm−2·s−1, thermal or epithermal, depending on the depth of the tumor, must be produced at the entrance of the patient.
Until a few years ago, before the advent of the accelerator-based neutron sources, the only device that is able to produce neutron sources with this intensity is fission nuclear reactor. Generally these sources were designed and realized at already existing reactors, designed for research or material testing purposes, and not for medical and BNCT applications. For this, BNCT irradiation beams have been realized using the existing horizontal or vertical channels in the reactor biological shield whose thickness increases with the reactor power. This limits the advantages coming from the use of high power reactors to have a more intense beam for BNCT due to the decrease of the neutron flux with the increase distance from the core to the beam exit. A way to overcome this is the use of a FCB installed in a position where an intense thermal neutron flux exists. This solution was used at MITR-II to realize an epithermal neutron beam (31,32). Moreover, it effectively reduced the photon contamination produced by neutron capture in the collimator.
In the design of a BNCT beam, beyond the intensity of neutron flux, some other characteristics must be taken into account with the main purpose to not reduce the BNCT selectivity.
Several parameters must be considered in the design of a BNCT beam, among which the most important are:
- The beam intensity to have reasonably short irradiation time, that is compatible with the comfort of the patient to keep a fixed position;
- The beam energy with high penetration capability to allow the treatment of deep seated tumors;
- The purity of the beam to not decrease the selectivity of the therapy that is the reduction of fast and thermal neutrons contamination, as well as that from photons.
A huge number of studies investigated the best ranges for these parameters. At the time in which these studies were carried out BNCT addressed primarily on brain tumors, thus the great majority of these studies used a water or polyethylene head phantom. The parameters to be considered are undoubtedly many, such as: the energy spectrum of the beam, the beam dimension, the collimation, the boron spatial distribution in healthy and cancer tissues (determined by the selectivity of the boron vector), the depth and position of the targeted tumour (33-40).
General guide values for this and other parameters were given for BNCT of brain tumors (41). Recommended values are:
- Fast neutrons contamination: <2.10−13 Gy cm2/n;
- Gamma contamination: <2.10−13 Gy cm2/n;
- Thermal to epithermal neutron flux ratio: <0.05;
- Neutron current to total flux ratio: >0.7;
- Beam radius: 12–15 cm.
Other suggested values reported for epithermal neutron beams (8) assuming borophenylalanine (BPA) as boron carrier and taking into account the radiation relative biological effectiveness (RBE) (1 for photons and 3.2 for neutrons) are as follows:
- Intensity: Φepi >2.109 cm−2·s−1;
- Energy: 0.4 eV < E ≤ 10–20 keV;
- Beam contamination (n+γ): <2.810−12 RBE Gy cm2;
- Beam collimation: J/Φ >0.75;
- Beam size and shape: adjustable beam size to at least 16 cm with possibilities to adjust beam shape;
- Treatment time: ~10 min per irradiation field;
- Patient and beam positioning: easy beam placement on any part of the body;
- Beam monitoring and control: accurate and reliable systems for neutron fluence delivery to ~1% of prescription;
- Treatment room: well shielded, easy and quick access, audio and visual communication with patients;
- Patient setup and support: separate spaces or rooms for exams, infusions, test setups and irradiation.
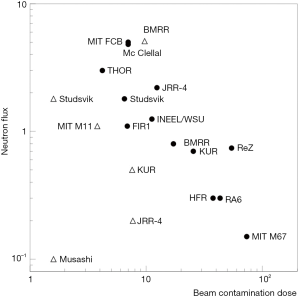
To give an idea of the performance of some epithermal and thermal BNCT beams, with respect to beam intensity and beam purity, Figure 2 reports the thermal and epithermal neutron flux at beam exit as a function of the beam contamination (dose from photons and fast neutrons in normal tissues) (8). The best beams are in the upper left-hand part of the figure.
The ability of a NCT beam to treat deep seated tumors depends on the dose rate profile in the patient body as a function of the depth. In BNCT, the total dose at a point includes many components: the boron dose, the proton dose (by thermal neutron capture in nitrogen and by fast neutron elastic scattering on hydrogen), gamma dose (by gamma contamination of the beam and by (n,γ) reactions (mainly on hydrogen) and inelastic scattering in tissues. The main components of the dose rate are related to the thermal neutron flux, so their profile is similar to that of thermal neutrons. Frequently the biologically weighted dose is used in place of the absorbed dose; in this case the used weighting parameters must be clearly specified. As an example, Figure 3 shows the depth dose rate profile along the beam axis as a function of depth in a head phantom for the MITR-II FCB epithermal beam (42); different components of BNCT dose and weighting factors are reported.
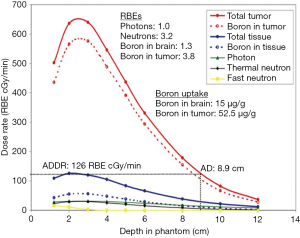
To evaluate the performance of an NCT beam for the treatment of a tumor, four parameters have been introduced: advantage depth (AD), advantage ratio (AR), advantage depth dose rate (ADDR) and the therapeutic gain (TG). AD is defined as the depth in the phantom at which the absorbed dose by the tumor is equal to the maximum dose delivered to healthy tissue; AR at a particular depth is defined as the ratio between the integral of tumor and normal tissue doses from the surface to the AD; ADDR is the dose rate at the AD, it is equal to the maximum dose rate in normal tissues and can be used to evaluate the irradiation time needed to reach the tolerance dose of normal tissue (33); finally, TG is defined as the ratio between the average dose in the tumor region and the maximum dose to normal tissues. In Figure 3, the AD and the ADDR of the beam are 8.9 cm and 126 RBE cGy/min, respectively.
All these parameters are very useful to design an NCT beam; Monte Carlo simulations (40) show that AD is almost independent by the boron concentration for neutron beams in the energy range from thermal up to 10 keV; while it is very sensitive at higher energies, for example at 100 keV. In the same paper (40), the AD as a function of neutron energy has been evaluated for three values of boron concentration (2, 10 and 40 ppm). A maximum value for the AD is reached in the region from few keV to a few tens of keV. Moreover the TG has been evaluated as a function of neutron energy (0.01 eV–1 MeV) for a brain tumour located at a depth of 5 cm and one at 8 cm. Again a maximum of this parameter is reached in the same energy range (few keV to a few tens of keV); showing that this is a good energy range for NCT beams. All simulations were done assuming a fixed value of the tumour/normal tissue concentration ratio of 4.3 and RBE factors of 1.6 and 2.3 for protons and 10B reactions respectively taken by Wallace et al. [1994] (38). As a general rule the TG depends on boron concentration and on the boron concentration ratio between tumor and healthy tissues; with higher boron concentrations and higher ratios, the higher values of TG are obtained.
Neutron beams and neutron field for BNCT
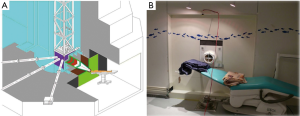
Neutrons produced by fission nuclear reactors have a typical spectrum with a most probable energy of 0.7 MeV, a mean energy of 2 MeV and a high energy tail up to about 10 MeV. Due to moderation process this neutron spectrum is changed in a new one with three main components: thermal, epithermal and fast. To produce a neutron beam useful for NCT of deep seated tumors some moderating and filtering materials are used to reduce the thermal and fast components and increase the epithermal one. Usually the principal materials are composed by Al, F, Mg because of the inelastic cross section behaviour characterized by an almost constant value at low energy and a series of resonances at energies higher than a few tens of keV; usually in a beam shaping assembly (BSA) Pb is used as neutrons reflector. In Figure 4, a view of the THOR and a photo of the treatment room are shown (7). A special case of BSA is represented at the Kyoto University Research Reactor Institute (KURRI) (14,15,41). The main part of the BSA is made by Al and D2O slabs; after this, further containers can be filled with D2O to shift the epithermal beam to lower energies adding a thermal component.
As last example we report a different approach, with respect to the reactor BNCT facilities cited until now, that was used at University of Pavia. With the aim to apply BNCT to the treatment of spread metastases invading a whole liver, a facility was realized at Triga Mark II reactor to treat an explanted organ. To have the possibility of irradiating the entire organ, not a beam but a neutron field was produced for modifying the existing thermal column to create a channel with the ability to host the explanted organ; a Bi layer was used to shield the gamma radiation coming from the core (24-26). Left panel of Figure 5 shows the irradiation position of the liver in the thermal column; the right panel shows the explanted liver (in the cylindrical Teflon container) was pushed into the thermal column for the irradiation.

Conclusions
In this article, we have reported a brief review of the fission reactor-based neutron sources for BNCT focusing on the main requirements which these sources are able to fulfil the BNCT application. General requirements for reactor BNCT neutron sources, techniques to produce epithermal neutron beams starting from fission neutrons and parameters for their performance evaluations have been briefly discussed.
There are some reactor-based BNCT centers still treating patients in Argentine, China, Japan and Taiwan. That will continue to give a fundamental contribution for further development of BNCT.
Surely accelerator-based BNCT sources, already built or under installation, will play a major role in the successful diffusion of BNCT as a standard cancer treatment; but the fission reactors have offered, until now, a unique opportunity to show the feasibility and effectiveness of BNCT on many techniques that will be useful for accelerator-based BNCT.
Moreover, nuclear reactors can be very useful to continue BNCT research for instrumentation development, boron concentration measurements in new boron compounds and in in vitro and in vivo experiments.
Acknowledgments
S Altieri is very grateful to Dr. Yi-Wei Chen and Dr. Hiroaki Kumada for kind invitation to write this paper.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hiroaki Kumada and Yi-Wei Chen) for the series “Boron Neutron Capture Therapy” published in Therapeutic Radiology and Oncology. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tro.2018.10.08). The series “Boron Neutron Capture Therapy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Harling OK, Riley KJ. 2012 Fission Reactor-Based Irradiation Facilities for Neutron Capture Therapy. In: Sauerwein WA, Wittig A, Moss R, et al. editors. Neutron Capture Therapy. Springer-Verlag Berlin Heidelberg, 2012;19-40.
- Ke G, Sun Z, Shen F, et al. The study of physics and thermal characteristics for in-hospital neutron irradiator (IHNI). Appl Radiat Isot 2009;67:S234-7. [Crossref] [PubMed]
- Zhang Z, Chong Y, Chen X, et al. PGNAA system preliminary design and measurement of in-hospital neutron irradiator for boron concentration measurement. Appl Radiat Isot 2015;106:161-5. [Crossref] [PubMed]
- Menéndez PR, Roth BMC, Pereira MD, et al. BNCT for skin melanoma in extremities: Updated Argentine clinical results Appl Radiat Isot 2009;67:S50-3. [Crossref] [PubMed]
- Yong Z, Song Z, Zhou Y, et al. Boron neutron capture therapy for malignant melanoma: first clinical case report in China. Chin J Cancer Res 2016;28:634-40. [Crossref] [PubMed]
- Sakurai Y, Tanaka H, Takata T, et al. Advances in boron neutron capture therapy (BNCT) at Kyoto University - From reactor-based BNCT to accelerator-based BNCT. J Korean Phys Soc 2015;67:76-81. [Crossref]
- Wang LW, Liu YWH, Chou FI, et al. Clinical trials for treating recurrent head and neck cancer with boron neutron capture therapy using the Tsing-Hua Open Pool Reactor. Cancer Commun (Lond) 2018;38:37. [Crossref] [PubMed]
- Harling OK, Riley KJ. Fission reactor neutron sources for neutron capture therapy - a critical review. J Neurooncol 2003;62:7-17. [Crossref] [PubMed]
- Moss RL, Stecher-Rasmussen F, Ravensberg K, et al. Design, construction and installation of an epithermal neutron beam for BNCT at the high flux reactor Petten. In: Allen BJ, Harrington BV, Moore DE. editors. Progress in Neutron Capture Therapy for Cancer. New York: Plenum Press, 1992;63-6.
- Skold K, Kierkegaard J, Gudowska I, et al. The Swedish facility for boron neutron capture therapy. Proceedings of the 9th International Symposium on Neutron Capture Therapy, Osaka, Japan, October 2-6, 2000;3940.
- Auterinen I, Hiismaiki P, Kotiluoto E, et al. Metamorphosis of a 35 year-old TRIGA reactor into a modern BNCT facility. In: Hawthorne MF, Wiersema RJ. editors. Frontiers in Neutron Capture Therapy, Vol. 1. New York: Kluwer Academic/Plenum Publishers, 2001;267-75.
- Marek M, Viererbl L, Burian J, et al. Determination of the geometric and spectral characteristics of BNCT beam (neutron and gamma-ray). In: Hawthorne MF, Wiersema RJ. editors. Frontiers in Neutron Capture Therapy, Vol. 1. New York: Kluwer Academic/Plenum Publishers, 2001;381-9.
- Marek M, Viererbl L, Flifbor S, et al. Validation of the epithermal neutron beam at LVR-15. Proceedings of the 9th International Symposium on Neutron Capture Therapy, Osaka, Japan, October 2-6, 2000;41-42.
- Sakurai Y, Kobayashi T, Kobayashi K. The characteristics of the updated heavy water facility of the Kyoto University Reactor (II) (neutron energy spectra for several irradiation modes). In: Hawthorne MF, Wiersema RJ. editors. Frontiers in Neutron Capture Therapy, Vol. 1. New York: Kluwer Academic/Plenum Publishers, 2001;345-349.
- Kobayashi T, Sakurai Y, Kanda K, et al. The remodeling and basic characteristics of the heavy water neutron irradiation facility of the Kyoto university research reactor, mainly for neutron capture therapy. Nucl Technol 2000;131:354-78. [Crossref]
- Yamamoto K, Kumada H, Torii Y, et al. Characteristics of neutron beams for BNCT. Proceedings of the 9th International Symposium on Neutron Capture Therapy, Osaka, Japan, October 2-6, 2000;243-244.
- Matsumoto T, Aizawa O, Nozaki T, et al. Present status of the medical irradiation facility at the Musashi reactor. Pigment Cell Res 1989;2:240-5. [Crossref] [PubMed]
- Aizawa O, Kand K, Nozaki T, et al. Remodeling and dosimetry on the neutron irradiation facility of the Musashi Institute of Technology Reactor for boron neutron capture therapy. Nucl Technol 1980;48:150-63. [Crossref]
- Liu HB, Razvi J, Rucker R, et al. TRIGA fuel-based converter assembly design for a dual-mode neutron beam system at the McClellan Nuclear Radiation Center. In: Hawthorne MF, Wiersema RJ. editors. Frontiers in Neutron Capture Therapy, Vol. 1. New York: Kluwer Academic/Plenum Publishers, 2001;295-300.
- Nigg DW, Wemple CA, Venhuizen JR, et al. Preliminary neutronic performance assessment of an epithermal neutron beam for preclinical BNCT Research at Washington State University. In: Venhuizen JR. editor. INEEL Advanced Radiotherapy Research Program Annual - Calendar Year 2001, INEEL/EXT-02-00060, 2002.
- Blaumann HR, Calzetta Larrieu O, Longhino JM, et al. NCT facility development and beam characterization at the RA-6 reactor. In: Hawthorne MF, Wiersema RJ. editors. Frontiers in Neutron Capture Therapy, Vol. 1. New York: Kluwer Academic/Plenum Publishers, 2001;313-7.
- Liu Y-WH, Teng YH, Liao MZ Design calculations of an epithermal neutron beam and development of a treatment planning system for the renovation of THOR for Boron Neutron Capture Therapy. Proceedings of the 9th International Symposium on Neutron Capture Therapy, Osaka, Japan, October 2-6, 2000;245-6.
- Burn KW, Colli V, Curzio G, et al. Characterization of the TAPIRO BNCT epithermal facility. Radiat Prot Dosimetry 2004;110:645-9. [Crossref] [PubMed]
- Pinelli T, Altieri S, Fossati F, et al. Development of a method to use boron neutron capture therapy for diffused tumors of liver (Taormina project). In: Mishima Y. editor. Neutron Capture Therapy for Cancer: Proc. 6th International Symposium (Kobe, Japan, 31 Oct-4 Nov 1994). New York: Plenum 1994;783-94.
- Pinelli T, Altieri S, Fossati F, et al. Operative modalities and effects of BNCT on liver metastases of colon adenocarcinoma. A microscopical and ultrastructural study in the rat. In: Hawthorne MF, Shelly K, Wiersema RJ. editors. Frontiers in Neutron Capture Therapy. New York: Kluwer Academic/Plenum Publishers, 1998;1427-40.
- Bortolussi S, Altieri S. Thermal neutron irradiation field design for boron neutron capture therapy of human explanted liver. Med Phys 2007;34:4700-5. [Crossref] [PubMed]
- Harling OK, Riley KJ, Newton TH, et al. The fission converter based epithermal neutron irradiation facility at the MIT Reactor. Nucl Sci Eng 2002;140:223-40. [Crossref]
- Rogus RD. Design and dosimetry of epithermal neutron beams for clinical trials of boron neutron capture therapy at the MITR-II Reactor. Ph.D. Thesis, Massachusetts Institute of Technology, 1994.
- Farr LE, Robertson JS, Stickley E. Neutron capture therapy with boron in the treatment of glioblastoma multiforme. Am J Roentgenol Radium Ther Nucl Med 1954;71:279-93. [PubMed]
- Slatkin DN. A history of boron neutron capture therapy of brain tumors. Brain 1991;114:1609-29. [Crossref] [PubMed]
- Harling OK, Riley KJ, Newton TH, et al. The new fission converter based epithermal neutron irradiation facility at MIT (INIS-XA-C--029). International Atomic Energy Agency (IAEA), 2001.
- Harling OK. Fission reactor based epithermal neutron irradiation facilities for routine clinical application in BNCT - Hatanaka memorial lecture. Appl Radiat Isot 2009;67:S7-11. [Crossref] [PubMed]
- Zamenhof RG, Murray BW, Brownell GL, et al. Boron neutron capture therapy for the treatment of cerebral gliomas. I: theoretical evaluation of the efficacy of various neutron beams. Med Phys 1975;2:47-60. [Crossref] [PubMed]
- Brugger RM, Constantine G, Harling OK, et al. Rapporteurs’ report. In: Harling OK, Bernard JA, Zamenhof RG. editors. Neutron beam, design, development and performance for neutron capture therapy. New York: Plenum Press, 1990;54.
- Clement SD, Choi JR, Zamenhof RG, et al. Monte Carlo methods of neutron beam design for neutron capture therapy at the MITR-II. In: Harling OK, Bernard JA, Zamenhof RG. editors. Neutron beam, design, development and performance for neutron capture therapy. New York: Plenum Press, 1990;51-70.
- Yanch JC, Zhou XL, Brownell GL. A Monte Carlo investigation of the dosimetric properties of monoenergetic neutron beams for neutron capture therapy. Radiat Res 1991;126:1-20. [Crossref] [PubMed]
- Yanch JC, Harling OK. Dosimetric effects of beam size and collimation of epithermal neutrons for boron neutron capture therapy. Radiat Res 1993;135:131-45. [Crossref] [PubMed]
- Wallace SA, Mathur JN, Allen BJ. Treatment planning figures of merit in thermal and epithermal boron neutron capture therapy of brain tumors. Phys Med Biol 1994;39:897-906. [Crossref] [PubMed]
- Sakamoto S, Kiger WS III, Harling OK. Sensitivity studies of beam directionality, beam size and neutron spectrum for a fission converter-based epithermal neutron beam for boron neutron capture therapy. Med Phys 1999;26:1979-88. [Crossref] [PubMed]
- Biscegliet E, Colangelo P, Colonna N, et al. On the optimal energy of epithermal neutron beams for BNCT. Phys Med Biol 2000;45:49-58. [Crossref] [PubMed]
- Current status of neutron capture therapy 2001 IAEA-TECDOC-1223.
- Research at the MIT NRL. Available online: http://web.mit.edu/nrl/www/bnct/info/description/descriptio.html. Accessed April 2, 2005.
Cite this article as: Altieri S, Protti N. A brief review on reactor-based neutron sources for boron neutron capture therapy. Ther Radiol Oncol 2018;2:47.


