
Complete response of unresectable icteric-type hepatocellular carcinoma to hypofractionated proton beam therapy with concurrent plus adjuvant sorafenib: a case report
Introduction
Icteric hepatocellular carcinoma (HCC) is an uncommon form of HCC, characterized by obstructive jaundice secondary to biliary tumor thrombi (1). The prognosis of icteric HCC is particularly dismal (2). According to a large retrospective cohort, the median overall survival (OS) was only 4 months, and most patients died of liver decompensation and biliary tract sepsis as a consequence of local tumor progression (3). En bloc resection of the tumor has long been the only treatment of choice conferring long-term survival, and cure through non-surgical approaches remains anecdotal (3-7). With modern advances in charged-particle radiotherapy and image-guidance technology, precise tailoring of ablative radiation dose to the hepatic tumor whilst minimizing the surrounding normal tissue damage has become achievable (8). The present report describes a successful outcome in an unresectable icteric HCC patient treated with state-of-the-art proton beam therapy (PBT) plus concurrent and adjuvant sorafenib.
Case presentation
A 68-year-old male patient with a history of hepatitis B carrier was admitted to a regional hospital because of progressive right upper abdominal pain, fatigue, jaundice, and recurrent biliary tract infection for 7 months. His total bilirubin was elevated to 5.9 mg/dL, and abdominal ultrasound showed dilatation of right intrahepatic ducts (IHD) and an irregular echo-bright lesion over right lobe perihilar region. Contrast-enhanced computed tomography (CT) revealed an infiltrative enhancing tumor (Figure 1A) located at segment 8 of the liver with extension into the right IHD and hilar region. A biopsy was performed, and moderately differentiated HCC was confirmed. After appropriate management of biliary tract infection, he was referred to our hospital for cancer management.
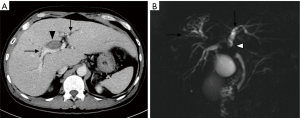
His laboratory tests showed the following results: alpha-fetoprotein (AFP) 4.1 ng/mL, CA19-9 465 U/mL, serum albumin 4.3 g/dL, total bilirubin 1.7 mg/dL, alkaline phosphatase (ALK-P) 126 IU/mL, AST 75 U/L, ALT 75 U/L, INR of 1.0, WBC 4,500/uL, Hb 12.2 g/dL and platelet 332,000/uL. Multiphasic gadolinium-ethoxybenzyl-diethylenetriamine penta-acetic acid (Gd-EOB-DTPA; Primovist, Bayer Healthcare Pharmaceuticals, Leverkusen, Germany)-enhanced magnetic resonance cholangiopancreatography (MRCP) was performed, which showed an infiltrative HCC at S8 with direct invasion into the segmental portal veins and common hepatic duct causing biliary tract dilatation (Figure 1B). His clinical cancer stage was T2N0M0 (7th edition of American Joint Committee on Cancer staging manual), with a Child-Pugh score of 5. The tumor board deemed the tumor to be unresectable, and proton beam radiotherapy with concurrent plus adjuvant sorafenib (Nexavar, Bayer Healthcare Pharmaceuticals, Leverkusen, Germany) was recommended. The patient was enrolled in the Sumitomo Proton Therapy System Post-Market Surveillance Study (protocol No. SHI-2016-01; Institutional Review Board No. 201600401B0) and signed the informed consent form.
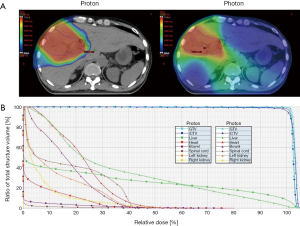
The patient was immobilized with a chest board and a customized alpha-cradle with arms abducted over the head. Abdominal belt compression was adopted to reduce respiratory organ motion. Four-dimensional and dynamic multiphasic contrast-enhanced CT images were acquired during the CT simulation. The gross tumor volume (GTV) and internal clinical target volume (iCTV) were 306 and 426 cm3, respectively. The normal liver volume (liver minus GTV) is 1,522 cm3. The patient received passive scattering PBT (Sumitomo Heavy Industries, Tokyo, Japan) using two oblique beams for 72.6 GyE in 22 fractions, five times per week, within 32 days (Figure 2A; dose-volume histogram, Figure 2B). Image-guided radiotherapy (IGRT) was performed via daily 2-dimentional kV orthogonal imaging to minimize the set-up uncertainty. Concurrent (200 mg) plus adjuvant (400 mg) sorafenib was administered twice daily.
Treatment-related acute toxicity included grade 3 dermatitis at high-dose area, grade 1 leukopenia (WBC 3,500/uL) and grade 1 thrombocytopenia (platelet 140,000/uL). At post-PBT 1-month, his total bilirubin, CA19-9, AST and ALT decreased to 0.7 mg/dL, 124.5 U/mL, 54 and 39 U/L, respectively. Other laboratory tests showed serum albumin 4.3 g/dL, AFP 6.5 ng/mL, ALK-P 95 U/L, INR of 1.1 and Hb 12.8 g/dL, and no change in the Child-Pugh score of 5. Post-PBT 4-month multiphasic Gd-EOB-DTPA-enhanced magnetic resonance imaging (MRI) revealed remarkable regression of irradiated tumor, and the delayed hepatobiliary phase images demonstrated a well-defined hypo-intense area that covered the hepatic tumor and was identical to the high-dose region of PBT (Figure 3A). The patient had a persistent skin ulcer of 2 cm × 1 cm inside the irradiated field and received surgical debridement. In consideration of excellent tumor control and his prolonged course of dermatitis healing, sorafenib was discontinued at post-PBT 6-month, and recovery of the skin ulcer was subsequently observed.
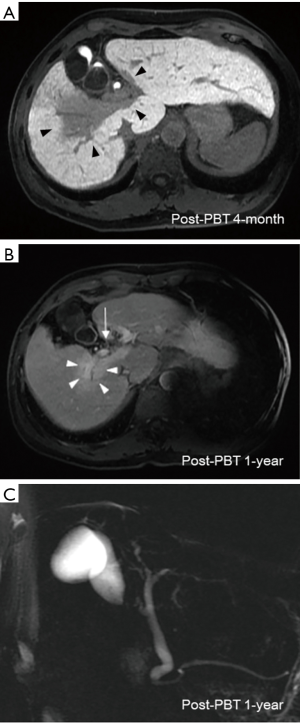
Laboratory tests taken at 6-month post-PBT showed improvement of liver function (serum albumin 4.49 g/dL, total bilirubin 0.5 mg/dL, AST 41 U/L, ALT 29 U/L, ALK-P 69 U/L and INR of 1.1) and CBC profile (WBC 4,400/uL, Hb 12.5 g/dL and platelet 189,000/uL). The CA19-9 declined to 51.7 U/mL, and AFP was 4.5 ng/mL. Multiphasic Gd-EOB-DTPA-enhanced MRI taken at post-PBT 1-year showed complete response of hepatic tumor according to the mRECIST guideline, and neither bile duct nor portal vein thrombi were visualized (Figure 3B). Regression of biliary tract dilatation was also observed (Figure 3C). After a follow-up of 27 months, the patient remained alive without evidence of recurrence.
Discussion
The management of icteric HCC remains a challenge, and en bloc surgical excision has long been the only hope of cure (9,10). Owing to the high frequency of hepatic hilum involvement and limited liver reserve, most patients are not amenable to curative resection (2-5). The prognosis of unresectable icteric HCC is discouraging, with a median survival ranging from 2 to 13 months (3,5-7). Due to the highly infiltrative nature of the disease, extensive tumor thrombi and hemobilia occluding the biliary system are frequently observed (11). Death is usually attributable to obstructive cholangitis and liver failure, as a result of local tumor proliferation (2-5).
For unresectable icteric-type HCC, liver-directed therapies are the most crucial elements of treatment in addition to proper biliary drainage (2-4). Various studies have shown that transarterial chemoembolization (TACE) with or without radiotherapy was associated with an OS improvement (3,5,7). However, due to the frequent vasculobiliary involvement, the efficacy of TACE for this clinical subtype is unsatisfactory (3,5,7). Furthermore, though photon-based radiotherapy has been increasingly incorporated into liver cancer management, the purpose of radiation treatment for icteric-type HCC, in general, remains palliative (3,5,7). Liver parenchyma is particularly vulnerable to radiation damage. In regard to the expansive tumor growth pattern and limited radiation tolerance of the liver, considerable dose reduction is usually required to meet the normal tissue constraints with conventional photon-based techniques, resulting in a suboptimal tumoricidal effect.
Distinct from the photon-based radiotherapy modalities, charged-particle radiation therapy including PBT and carbon ion beam therapy (CIBT) offers unique dosimetric advantages allowing a finite range of dose delivery with an abrupt dose falloff beyond the beam paths. With the dosimetric advantages, ablative radiation dose can be precisely delivered to the tumor whilst decreasing the surrounding normal tissue exposure (12,13). In the published literature, the local control (LC) rate in patients with HCC undergoing hypofractionated PBT or CIBT was up to 80–95% at 3–5 years (8,12-18). In the present report, the dose fractionation schedule of 72.6 GyE in 22 fractions was consistent with the Tsukuba PBT protocol (15,17). We performed a dosimetric comparison of PBT versus photon-based volumetric modulated arc therapy (VMAT) using TrueBeam System and jaw-tracking technology (Varian Medical Systems, California, USA). Using the same dose prescription criteria, VMAT produces a low dose radiation bath to a greater volume of liver parenchyma (V5: VMAT, 1,398 mL versus PBT, 624 mL; Figure 2A), resulting in a remarkably higher mean liver dose (VMAT, 26.1 Gy versus PBT, 19.5 Gy). The dose-volume histogram also demonstrated higher cumulative doses of bowel, heart, spinal cord and kidneys in the VMAT plan, compared with passive scattering PBT plan (Figure 2B). These dosimetric advantages have also been reflected in the clinical outcomes. Since Gd-EOB-DTPA contrast is taken up and then excreted into the biliary system by functional hepatocytes, the radiation-associated hepatocyte dysfunction can be explicitly depicted by a hypo-intense imaging presentation on the hepatobiliary phase images (19). Our Gd-EOB-DTPA-enhanced MRI clearly visualized a distinct hypo-intense area which was exactly co-localized at the high-dose region of PBT and contrasted with a well-enhanced hyper-intense background of unirradiated liver (Figure 3A), suggesting that PBT specifically prevented a large volume of liver parenchyma from unnecessary low-dose radiation insults. Furthermore, neither liver function deterioration nor any bowel, heart, spinal cord and renal toxicity was documented during the 14 months of the patient follow-up, indicating that minimizing radiation exposure of normal organs might be clinically relevant. More importantly, the effective irradiated volume of liver in the VMAT plan was 36%. To safely execute the VMAT plan, the prescription dose had to be reduced to 39 Gy in 6 fractions to meet the normal organ constraints for high-precision stereotactic body radiotherapy (SBRT) (20). The dosimetric superiority of PBT widened the therapeutic window and permitted the patient, who presented with a centrally-located infiltrative icteric-type HCC, to undergo ablative radiation treatment without jeopardizing the surrounding critical organs.
As reported in a recent multi-institutional phase II study, the 2-year LC rate was 94.8% in HCC patients treated with high-dose hypofractionated PBT, and a favorable 2-year OS rate of 63.2% (21), suggesting that dose-escalated PBT is highly effective in the eradication of HCC. Consistent with published results, the present case demonstrated an imaging complete response and without evidence of tumor recurrence. Intriguingly, though the patient did not receive biliary drainage, post-PBT MRI showed normalization of bile duct dilatation concomitant with a gradual recovery in the liver function tests and total bilirubin levels, suggesting that controlling the icteric-type HCC was associated with restoration of biliary tract patency. Notwithstanding the poor prognosis of icteric-type HCC patients, when the biliary obstruction is properly managed, outcomes are generally better than HCC patients who have jaundice resulting from hepatic parenchymal insufficiency (4). Consequently, our observations further highlight the importance of local tumor control in achieving a sustainable relief of biliary tract obstruction and prolonging survival.
Since tumor invasion of vasculobiliary system was evident, the patient is at high risk for systemic cancer metastases, indicating the use of sorafenib (22). According to a prospective phase II study, the combination of SBRT and concurrent (400 mg twice daily) plus 6-month adjuvant (400 mg twice daily) sorafenib might increase the radiosensitivity of the tumor, despite the fact that augmentation of hepatic toxicity was also observed in such combined-modality treatment (23). Unlike the photon-based SBRT technology, PBT provided a substantial sparing of liver tissue from low-dose radiation exposure, minimizing the synergistic damage of sorafenib and scattered radiation in a large portion of liver parenchyma. In the present case, we prescribed half (200 mg twice daily) of the recommended dose of sorafenib during the PBT, and no hepatic complication was observed after the combined treatment. Nonetheless, we documented a prolonged course of grade 3 dermatitis. It has been widely reported that sorafenib frequently causes dermatological complications and impairs wound healing process (22-24), and it may increase the radiation toxicity of skin tissue. Furthermore, PBT deposited a higher entrance dose on the irradiated skin as compared to megavoltage X-ray resulting in a greater risk of radiation dermatitis (8,21). Taken together, these factors might potentially explain the prolonged course of skin adverse event. Though the dermatological toxicity in the present case remained manageable, caution is needed when such combined treatment is adopted.
In conclusion, unresectable icteric-type HCC has long been regarded as an incurable disease. The present report, for the first time, described a successful treatment result with a favorable toxicity profile in a centrally-located icteric-type HCC patient using high-dose hypofractionated PBT with concurrent plus adjuvant sorafenib. Further prospective studies are urgently needed to validate the effectiveness of PBT in this clinical entity.
Acknowledgments
Funding: This study is supported by the grant CIRPG3D0141 from Chang Gung Memorial Hospital.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lin TY, Chen KM, Chen YR, et al. Icteric type hepatoma. Med Chir Dig 1975;4:267-70. [PubMed]
- Chen MF. Icteric type hepatocellular carcinoma: clinical features, diagnosis and treatment. Chang Gung Med J 2002;25:496-501. [PubMed]
- Suh YG, Kim DY, Han KH, et al. Effective biliary drainage and proper treatment improve outcomes of hepatocellular carcinoma with obstructive jaundice. Gut Liver 2014;8:526-35. [Crossref] [PubMed]
- Qin LX, Tang ZY. Hepatocellular carcinoma with obstructive jaundice: diagnosis, treatment and prognosis. World J Gastroenterol 2003;9:385-91. [Crossref] [PubMed]
- Lai EC, Lau WY. Hepatocellular carcinoma presenting with obstructive jaundice. ANZ J Surg 2006;76:631-6. [Crossref] [PubMed]
- Lau WY, Leung KL, Leung TW, et al. Obstructive jaundice secondary to hepatocellular carcinoma. Surg Oncol 1995;4:303-8. [Crossref] [PubMed]
- Huang JF, Wang LY, Lin ZY, et al. Incidence and clinical outcome of icteric type hepatocellular carcinoma. J Gastroenterol Hepatol 2002;17:190-5. [Crossref] [PubMed]
- Skinner HD, Hong TS, Krishnan S. Charged-Particle Therapy for Hepatocellular Carcinoma. Semin Radiat Oncol 2011;21:278-86. [Crossref] [PubMed]
- Peng BG, Liang LJ, Li SQ, et al. Surgical treatment of hepatocellular carcinoma with bile duct tumor thrombi. World J Gastroenterol 2005;11:3966-9. [Crossref] [PubMed]
- Meng KW, Dong M, Zhang WG, et al. Clinical Characteristics and Surgical Prognosis of Hepatocellular Carcinoma with Bile Duct Invasion. Gastroenterol Res Pract 2014;2014:604971 [Crossref] [PubMed]
- Lau WY, Leow CK, Leung KL, et al. Cholangiographic Features in the Diagnosis and Management of Obstructive Icteric Type Hepatocellular Carcinoma. HPB Surg 2000;11:299-306. [Crossref] [PubMed]
- Dionisi F, Widesott L, Lorentini S, et al. Is there a role for proton therapy in the treatment of hepatocellular carcinoma? A systematic review. Radiother Oncol 2014;111:1-10. [Crossref] [PubMed]
- Kasuya G, Kato H, Yasuda S, et al. Progressive hypofractionated carbon-ion radiotherapy for hepatocellular carcinoma: Combined analyses of 2 prospective trials. Cancer 2017;123:3955-65. [Crossref] [PubMed]
- Bush DA, Kayali Z, Grove R, et al. The safety and efficacy of high-dose proton beam radiotherapy for hepatocellular carcinoma: a phase 2 prospective trial. Cancer 2011;117:3053-9. [Crossref] [PubMed]
- Mizumoto M, Okumura T, Hashimoto T, et al. Proton Beam Therapy for Hepatocellular Carcinoma: A Comparison of Three Treatment Protocols. Int J Radiat Oncol Biol Phys 2011;81:1039-45. [Crossref] [PubMed]
- Komatsu S, Fukumoto T, Demizu Y, et al. Clinical results and risk factors of proton and carbon ion therapy for hepatocellular carcinoma. Cancer 2011;117:4890-904. [Crossref] [PubMed]
- Nakayama H, Sugahara S, Tokita M, et al. Proton beam therapy for hepatocellular carcinoma: the University of Tsukuba experience. Cancer 2009;115:5499-506. [Crossref] [PubMed]
- Chiba T, Tokuuye K, Matsuzaki Y, et al. Proton Beam Therapy for Hepatocellular Carcinoma: A Retrospective Review of 162 Patients. Clin Cancer Res 2005;11:3799-805. [Crossref] [PubMed]
- Yuan Y, Andronesi OC, Bortfeld TR, et al. Feasibility study of in vivo MRI based dosimetric verification of proton end-of-range for liver cancer patients. Radiother Oncol 2013;106:378-82. [Crossref] [PubMed]
- Bujold A, Massey CA, Kim JJ, et al. Sequential Phase I and II Trials of Stereotactic Body Radiotherapy for Locally Advanced Hepatocellular Carcinoma. J Clin Oncol 2013;31:1631-9. [Crossref] [PubMed]
- Hong TS, Wo JY, Yeap BY, et al. Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients With Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J Clin Oncol 2016;34:460-8. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in Advanced Hepatocellular Carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Chen SW, Lin LC, Kuo YC, et al. Phase 2 Study of Combined Sorafenib and Radiation Therapy in Patients With Advanced Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys 2014;88:1041-7. [Crossref] [PubMed]
- Brose MS, Frenette CT, Keefe SM, et al. Management of Sorafenib-Related Adverse Events: A Clinician’s Perspective. Semin Oncol 2014;41:S1-16. [Crossref] [PubMed]
Cite this article as: Hsieh CE, Hong JH, Lin CC, Lee CH, Hung SP, Chou CY, Chen CM, Tseng JH. Complete response of unresectable icteric-type hepatocellular carcinoma to hypofractionated proton beam therapy with concurrent plus adjuvant sorafenib: a case report. Ther Radiol Oncol 2018;2:42.


