
Prognostic factors of axillary lymph node-positive patients in clinical stage II and III breast cancer after neoadjuvant chemotherapy
Introduction
Surgery following neoadjuvant chemotherapy (NAC) is increasingly utilized for breast cancer treatment for various reasons. First, NAC results in loco-regional downstage and promotes breast conserving surgery (BCS) for better cosmetic outcomes (1-3). Second, the chemotherapeutic response can be tested before tumor excision via this approach, which helps avoid any ineffective adjuvant or NAC regimens and their resultant toxicities. In other words, earlier systemic treatment with effective regimens helps reduce risk of recurrence. Third, NAC allows selection of the optimal treatment option for individualized patient subgroups based on their therapeutic response and prognostic risks. However, as compared to the effectiveness of performing adjuvant radiotherapy after surgery, this approach involves uncertainties regarding the solutions of adjuvant radiotherapy (design of RT field and indication in low risk patients) in patients receiving NAC [e.g., the indication of treatment coverage of the axilla region regarding the dissected lymph nodes and post-mastectomy radiotherapy (PMRT) for low-risk patients with complete response]. In the past, most recurrent risks indicated by high-level evidence were those patients receiving adjuvant chemotherapy and radiotherapy following primary surgery. However, till date, when originally applied in patients with locally advanced disease, NAC has subsequently presented its extended indications even in the early stage disease.
Good response after NAC [e.g., pathological complete response (pCR)] is a strong predictor of its favorable outcome (1,4-6). The predictors reported in previous studies on primary surgery may not be accurate for assessing recurrent risks which are required to make decisions regarding loco-regional radiotherapy.
The impact of axillary lymph node status and correlated risk factors on the loco-regional events is also not well documented. Even the National Comprehensive Cancer Network (NCCN) guidelines of 2018.V1 state that “treat any part of axillary bed at risk” without interpretation of the types of risks in detail. The objective of this study was to identify the risk factors of loco-regional recurrence (LRR), relapse (any local, regional, or distant recurrence) and the overall survival (OS) with respect to tumor response after NAC in patients treated at our institution.
Methods
Patient characteristics
We retrospectively reviewed the patients treated at our institute between 2007 and 2015; 190 non-metastatic patients had received neoadjuvant systemic treatment, surgery, and adjuvant radiotherapy. The exclusion criteria were previous breast cancer disease (N=1), synchronous bilateral breast cancer disease (N=1), non-pure infiltrating ductal carcinoma (N=6), neoadjuvant treatment by hormone therapy alone (N=2), no adjuvant radiotherapy in this institute (N=2), no follow-up (N=3), human epidermal growth factor receptor 2 (HER-2) positive patients without trastuzumab (Herceptin®) prescription (N=6) and no residual lymph node disease (N=79). Patients were considered positive for HER-2 when there was a strong over-expression of HER2 immunohistochemical study (3+) or gene amplification by fluorescence in situ hybridization (FISH) in samples obtained by biopsy or definitive surgery.
In total, 90 patients were found eligible for the final analysis in this study. The patient characteristics included diagnosed at age ≤/>50 years, surgery method as BCS or total mastectomy, clinical T/N categories and stage, pathological T/N categories, therapeutic response after NAC, the number and positive lymph nodes ratio, tumor grading, surgical margin (≤/> 1 mm), the presence or absence of extracapsular extension (ECE), the presence or absence of lymphovascular invasion (LVI), tumor biomarker status [such as estrogen receptor (ER)/ progesterone receptor (PR)/HER-2], and adjuvant chemotherapy/target therapy.
Diagnostic method and operation details
The initial assessment of axillary disease was performed by ultrasound or aspiration cytology of the lymph nodes. BCS or total mastectomy with axillary lymph node dissection (ALND) and/or sentinel lymph node biopsy (SLNB) was performed by surgeons according to the institute’s guidelines for treating breast cancer.
Systemic therapy
According to the clinical practice guidelines of this institute, neoadjuvant anthracycline-based chemotherapy with or without taxane was administered for 2–6 cycles (most commonly 4 cycles). The decision whether adjuvant chemotherapy should be administrated depended on previous incomplete neoadjuvant cycles and response after NAC, and the total neoadjuvant and adjuvant chemotherapy course comprised 8 cycles. Trastuzumab (Herceptin®) every 3 weeks for a total duration of a year in neoadjuvant and adjuvant periods was prescribed for patients with strong over-expression or gene amplification of HER2 status. All patients received surgery after an NAC course of 2–6 weeks. Hormone therapies of tamoxifen, aromatase inhibitor, or GnRH inhibitor were prescribed for patients with positive ER or PR status for at least 5 years after adjuvant radiotherapy.
Response assessment
We compared the initial tumor size by ultrasound (preferred) or MRI (in case of no ultrasound), which were assessed by a certified surgical oncologist or radiologist, and the post-treatment tumor size was assessed by final pathological report. The response subgroups were defined as complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD) with reference to the response evaluation criteria in solid tumor (RECIST 1.1) as a reference (7). CR was defined as no residual invasive disease, such as ypT0/Tis and ypN0 (6).
Radiotherapy technique
Adjuvant radiotherapy for the whole breast or chest wall with lymphatic areas [supra/infraclavicular fossae (SCF/ICF) and internal mammary chain (IMC)] was performed using 2D-radiotherapy (2DRT), 3D-conformal RT (3DCRT), intensity-modulated radiotherapy (IMRT), or volumetric arc therapy (VMAT). For patients undergoing BCS, a total radiation dose of 45–50.4 Gy (1.8–2.0 Gy daily fraction) was delivered to the whole breast, with or without regional lymphatic areas (SCF/ICF and IMC), followed by a 10–16 Gy boost to the tumor bed. For patients undergoing total mastectomy, a total radiation dose of 50–50.4 Gy (1.8–2.0 Gy per fraction) was delivered to the chest wall, with or without regional lymphatic areas (SCF/ICF and IMC). The patients who had close margin (≤1 mm) and/or skin invasion in the mastectomy group were allowed to receive tumor bed boost with 10–16 Gy. All patients with the axillary disease after NAC received RT to the regional lymphatic areas (SCF, ICF, and IMC).
Statistical analysis
The Kaplan-Meier survival analysis was performed and compared by log-rank test. Univariate and multivariate analyses for LRR, relapse-free survival (RFS), and OS using Cox-proportional hazard model were performed to estimate the hazard ratio (HR) and 95% confidence interval (CI). RFS is defined as any disease recurrence (local, regional, or distant); however, death was censored (data not shown). P value ≤0.05 was considered to indicate statistical significance. Statistical analyses were calculated using the IBM SPSS Statistics for MAC (Version 22.0 IBM Corp., Armonk, NY).
Institutional Review Board (IRB)
The study was approved by the Breast Cancer Multidiscipline Group of Changhua Christian Hospital (CCH), and the ethical approval for the same was obtained from the committee on human experimentation of the same institution (CCH IRB No. 180313).
Results
Patients
A total of 90 patients with clinical stages II and III invasive ductal carcinoma received neoadjuvant anthracycline-based chemotherapy with or without taxane and target therapy, followed by curative surgery and adjuvant radiotherapy. With a median follow-up duration of 62 months (7–125 months), the median age at diagnosis was 49 years (25–76 years) in the entire cohort. Of these patients, 44 (49%) women had clinical stage II disease and 46 (51%) had clinical stage III disease. The subgroups on the basis of clinical lymph node status included: N0, N1 micro/N1, N2, and N3, which were observed in 11 (12%), 57 (63%), 16 (18%), and 6 (7%) patients. The number and percentage of women with BCS, diagnosed at >50 years, primary tumor at the left side, and the primary tumor location at UOQ were 24 (27%), 37 (41%), 42 (47%) and 39 (43%), respectively. The numbers and percentages of the factors such as <10 dissected lymph nodes, surgical margin of >1 mm, presence of lymphovascular invasion and presence of extracapsular extension of lymph nodes were 19 (21%), 67 (74%), 71 (79%) and 50 (56%), respectively, as per the final pathological report. The numbers of patients with positive lymph nodes ratio of 0–33%, 34–66% and 67–100% were 52 (58%), 20 (22%) and 18 (20%), respectively.
After NAC, the comparison of initial and post-treatment size demonstrated PR, SD, and PD in 52 (58%), 28 (31%) and 10 (11%) patients, respectively. The tumor types categorized by ER/PR/HER2 status, referred to as luminal A/B, Her2 positive and triple-negative breast cancer (TNBC), were observed in 50 (56%), 29 (32%), and 11 (12%) patients, respectively. There were 39 (43%) patients receiving the adjuvant chemotherapy and/or target therapy. Patient characteristics are summarized in Table 1.
Table 1
| Characteristics | N | % |
|---|---|---|
| Age (years) | ||
| ≤50 | 53 | 59 |
| >50 | 37 | 41 |
| Surgery | ||
| BCS | 24 | 27 |
| Mastectomy | 66 | 73 |
| Tumor laterality | ||
| Left | 42 | 47 |
| Right | 48 | 53 |
| Tumor location | ||
| UOQ | 39 | 43 |
| Non-UOQ | 51 | 57 |
| Clinical stage | ||
| c stage II | 44 | 49 |
| c stage III | 46 | 51 |
| Clinical T | ||
| cT1–2 | 51 | 57 |
| cT3 | 24 | 27 |
| cT4 | 15 | 17 |
| Clinical N | ||
| cN0 | 11 | 12 |
| cN1 micro/N1 | 57 | 63 |
| cN2 | 16 | 18 |
| cN3 | 6 | 7 |
| Response | ||
| Partial | 52 | 58 |
| Stable | 28 | 31 |
| Progression | 10 | 11 |
| Pathological T | ||
| T1–2 | 10 | 11 |
| T3 | 53 | 59 |
| T4 | 27 | 30 |
| Pathological N | ||
| N0ihc/mi/1A | 51 | 57 |
| N2a | 29 | 32 |
| N3a | 10 | 11 |
| Dissected lymph nodes | ||
| 1–3# | 3 | 3 |
| 4–9# | 16 | 18 |
| ≥10# | 71 | 79 |
| LN positive ratio | ||
| 0–33% | 52 | 58 |
| 34–66% | 20 | 22 |
| 67–100% | 18 | 20 |
| Grade | ||
| ≤1 | 19 | 21 |
| 2 | 49 | 54 |
| 3 | 22 | 24 |
| Surgical margin (mm) | ||
| >1 | 67 | 74 |
| ≤1 | 23 | 26 |
| Lymphovascular invasion | ||
| No | 19 | 21 |
| Yes | 71 | 79 |
| Extracapsular extension | ||
| No | 40 | 44 |
| Yes | 50 | 56 |
| Tumor type | ||
| ER/PR+ Her2− | 50 | 56 |
| ER/PR+ Her2+ | 20 | 22 |
| ER/PR− Her2+ | 9 | 10 |
| TNBC | 11 | 12 |
| Adjuvant chemotherapy or target therapy | ||
| No | 51 | 57 |
| Yes | 39 | 43 |
BCS, breast conserving surgery; LN, lymph nodes; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor type2; TNBC, triple negative breast cancer.
Outcome and prognostic significance
Univariate analysis of risks of LRR showed that clinical N category (cN3: HR =8.43, P=0.015), therapeutic response after NAC (SD: HR =2.54, P=0.038; PD: HR =5.32, P=0.002), dissected lymph nodes (≥10 nodes: HR =0.37, P=0.018) and positive lymph nodes ratio (67–100%: HR =5.14, P<0.001) are statistically significant. Multivariate analysis of risks of LRR also showed that the status of clinical lymph nodes (cN2: HR =6.07, P=0.046; cN3: HR =30.22, P=0.001), response subgroups (SD: HR =3.01, P=0.047; PD: HR =10.76, P<0.001), and positive lymph nodes ratio (67–100%: HR =4.32, P=0.025) are statistically significant (Table 2).
Table 2
| Characteristics | Total | LRR | Univariate analysis | Multivariate analysis (adjusted) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | HR | 95% CI | P value | HR | 95% CI | P value | ||||||
| Lower | Upper | Lower | Upper | ||||||||||
| Age (years) | |||||||||||||
| ≤50 | 53 | 13 | 24.5 | 1 | |||||||||
| >50 | 37 | 13 | 35.1 | 1.26 | 0.86 | 1.86 | 0.236 | ||||||
| Surgery | |||||||||||||
| BCS | 24 | 6 | 25.0 | 1 | |||||||||
| Mastectomy | 66 | 20 | 30.3 | 1.37 | 0.55 | 3.41 | 0.500 | ||||||
| Clinical stage | |||||||||||||
| c stage II | 44 | 13 | 29.5 | 1 | |||||||||
| c stage III | 46 | 13 | 28.3 | 0.92 | 0.43 | 1.98 | 0.830 | ||||||
| Clinical T | |||||||||||||
| cT1–2 | 51 | 13 | 25.5 | 1 | |||||||||
| cT3 | 24 | 8 | 33.3 | 1.17 | 0.49 | 2.83 | 0.725 | ||||||
| cT4 | 15 | 5 | 33.3 | 1.34 | 0.48 | 3.77 | 0.574 | ||||||
| Clinical N | |||||||||||||
| cN0 | 11 | 2 | 18.2 | 1 | 1 | ||||||||
| cN1 micro/N1 | 57 | 13 | 22.8 | 1.44 | 0.33 | 6.40 | 0.630 | 3.27 | 0.61 | 17.63 | 0.168 | ||
| cN2 | 16 | 7 | 43.8 | 3.14 | 0.65 | 15.20 | 0.154 | 6.07 | 1.04 | 35.61 | 0.046* | ||
| cN3 | 6 | 4 | 66.7 | 8.43 | 1.51 | 47.21 | 0.015* | 30.22 | 4.27 | 213.62 | 0.001* | ||
| Response | |||||||||||||
| Partial | 52 | 9 | 18.3 | 1 | 1 | ||||||||
| Stable | 28 | 11 | 39.3 | 2.54 | 1.05 | 6.13 | 0.038* | 3.01 | 1.02 | 8.92 | 0.047* | ||
| Progression | 10 | 6 | 60.0 | 5.32 | 1.88 | 15.00 | 0.002* | 10.76 | 3.14 | 36.86 | <0.001** | ||
| Pathological T | |||||||||||||
| T1–2 | 10 | 2 | 20.0 | 1.00 | |||||||||
| T3 | 53 | 13 | 24.5 | 1.24 | 0.28 | 5.50 | 0.778 | ||||||
| T4 | 27 | 11 | 40.7 | 2.35 | 0.52 | 10.61 | 0.267 | ||||||
| Pathological N | |||||||||||||
| N0ihc/mi/1A | 51 | 12 | 23.5 | 1 | 1 | ||||||||
| N2a | 29 | 9 | 31.0 | 1.44 | 0.61 | 3.42 | 0.409 | 0.48 | 0.16 | 1.46 | 0.197 | ||
| N3a | 10 | 5 | 50.0 | 2.63 | 0.92 | 7.49 | 0.071 | 0.58 | 0.15 | 2.23 | 0.423 | ||
| Dissected lymph node | |||||||||||||
| <10# | 19 | 9 | 47.4 | 1 | |||||||||
| ≥10# | 71 | 17 | 23.9 | 0.37 | 0.17 | 0.84 | 0.018* | ||||||
| LN+ ratio | |||||||||||||
| 0–33% | 52 | 9 | 17.3 | 1 | 1.00 | ||||||||
| 34–66% | 20 | 6 | 30.0 | 2.09 | 0.74 | 5.89 | 0.162 | 2.17 | 0.59 | 7.98 | 0.242 | ||
| 67–100% | 18 | 11 | 1.1 | 5.14 | 2.11 | 12.50 | <0.001** | 4.32 | 1.20 | 15.54 | 0.025* | ||
| Grade | |||||||||||||
| ≤1 | 19 | 3 | 15.8 | 1 | |||||||||
| 2 | 49 | 16 | 32.7 | 2.30 | 0.67 | 7.89 | 0.186 | ||||||
| 3 | 22 | 7 | 31.8 | 2.65 | 0.68 | 10.24 | 0.159 | ||||||
| Surgical margin (mm) | |||||||||||||
| >1 | 67 | 20 | 29.9 | 1 | |||||||||
| ≤1 | 23 | 6 | 26.1 | 0.84 | 0.34 | 2.10 | 0.713 | ||||||
| Lymphovascular invasion | |||||||||||||
| No | 19 | 4 | 21.1 | 1 | |||||||||
| Yes | 71 | 22 | 31.0 | 1.59 | 0.55 | 4.60 | 0.397 | ||||||
| Extracapsular extension | |||||||||||||
| No | 40 | 11 | 27.5 | 1 | |||||||||
| Yes | 50 | 15 | 30.0 | 1.07 | 0.49 | 2.33 | 0.868 | ||||||
| Tumor type | |||||||||||||
| ER/PR+ Her2− | 50 | 15 | 30.0 | 1 | |||||||||
| ER/PR+ Her2+ | 20 | 5 | 25.0 | 0.59 | 0.20 | 1.79 | 0.352 | ||||||
| ER/PR− Her2+ | 9 | 2 | 22.2 | 0.49 | 0.13 | 1.84 | 0.291 | ||||||
| TNBC | 11 | 4 | 36.4 | 0.42 | 0.08 | 2.31 | 0.320 | ||||||
| Adjuvant chemotherapy or target therapy | |||||||||||||
| No | 51 | 14 | 27.5 | 1 | |||||||||
| Yes | 39 | 12 | 30.8 | 1.29 | 0.60 | 2.79 | 0.519 | ||||||
*, indicates P<0.05; **, indicates P<0.001. LRR, loco-regional recurrence; HR, hazard ratio; BCS, breast conserving surgery; LN+ ratio, positive ratio of dissected lymph nodes; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor type2; TNBC, triple negative breast cancer.
Univariate analysis of risks of relapse also showed that the clinical N category (cN3: HR =6.73, P=0.015), therapeutic response after NAC (PD: HR =3.09, P=0.012), pathological N category (pN3a: HR =2.72, P=0.025), and positive lymph nodes ratio (67%–100%: HR =3.65, P<0.001) are statistically significant. The clinical N categories (cN1 micro/N1: HR =3.97, P=0.037, cN3: HR =10.39, P=0.005), therapeutic response after NAC (PD: HR =3.73, P=0.008) and positive lymph nodes ratio (67–100%: HR =3.02, P=0.032) also yielded statistical significance in the multivariate analysis of risks of relapse. Moreover, the clinical N category cN2 showed a statistical trend (HR =4.06, P=0.053) (Table 3).
Table 3
| Characteristics | Total | Relapse | Univariate analysis | Multivariate analysis (adjusted) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | HR | 95% CI | P value | HR | 95% CI | P value | ||||||
| Lower | Upper | Lower | Upper | ||||||||||
| Age (years) | |||||||||||||
| ≤50 | 53 | 23 | 43.4 | 1 | |||||||||
| >50 | 37 | 18 | 48.6 | 1.13 | 0.827 | 1.543 | 0.445 | ||||||
| Surgery | |||||||||||||
| BCS | 24 | 9 | 37.5 | 1 | |||||||||
| Mastectomy | 66 | 32 | 48.5 | 1.51 | 0.721 | 3.177 | 0.274 | ||||||
| Clinical stage | |||||||||||||
| c stage II | 44 | 22 | 50.0 | 1 | |||||||||
| c stage III | 46 | 19 | 41.3 | 0.8 | 0.434 | 1.486 | 0.484 | ||||||
| Clinical T | |||||||||||||
| cT1–2 | 51 | 24 | 47.1 | 1 | |||||||||
| cT3 | 24 | 11 | 45.8 | 0.84 | 0.409 | 1.708 | 0.622 | ||||||
| cT4 | 15 | 6 | 40.0 | 0.83 | 0.339 | 2.035 | 0.685 | ||||||
| Clinical N | |||||||||||||
| cN0 | 11 | 3 | 27.3 | 1 | 1 | ||||||||
| cN1 micro/N1 | 57 | 26 | 45.6 | 2.13 | 0.636 | 7.113 | 0.22 | 3.97 | 1.08 | 14.51 | 0.037* | ||
| cN2 | 16 | 8 | 50.0 | 2.63 | 0.684 | 10.1 | 0.159 | 4.06 | 0.98 | 16.8 | 0.053*** | ||
| cN3 | 6 | 4 | 66.7 | 6.73 | 1.457 | 31.11 | 0.015* | 10.39 | 1.99 | 54.33 | 0.005* | ||
| Response | |||||||||||||
| Partial | 52 | 18 | 34.6 | 1 | 1 | ||||||||
| Stable | 28 | 13 | 57.1 | 1.79 | 0.908 | 3.516 | 0.093 | 1.49 | 0.65 | 3.42 | 0.349 | ||
| Progression | 10 | 7 | 70.0 | 3.09 | 1.285 | 7.423 | 0.012* | 3.73 | 1.42 | 9.83 | 0.008* | ||
| Pathological T | |||||||||||||
| T1–2 | 10 | 5 | 50.0 | 1 | |||||||||
| T3 | 53 | 22 | 41.5 | 0.83 | 0.313 | 2.193 | 0.705 | ||||||
| T4 | 27 | 14 | 51.9 | 1.17 | 0.42 | 3.25 | 0.766 | ||||||
| Pathological N | |||||||||||||
| N0ihc/mi/1A | 51 | 18 | 35.3 | 1 | 1 | ||||||||
| N2a | 29 | 16 | 55.2 | 1.75 | 0.89 | 3.433 | 0.105 | 1.07 | 0.46 | 2.49 | 0.867 | ||
| N3a | 10 | 7 | 70.0 | 2.72 | 1.131 | 6.554 | 0.025* | 0.94 | 0.29 | 3.06 | 0.913 | ||
| Dissected lymph node | |||||||||||||
| <10# | 19 | 9 | 47.4 | 1 | |||||||||
| ≥10# | 71 | 32 | 45.1 | 0.73 | 0.346 | 1.535 | 0.405 | ||||||
| LN+ ratio | |||||||||||||
| 0–33% | 52 | 18 | 34.6 | 1 | 1 | ||||||||
| 34–66% | 20 | 9 | 45.0 | 1.73 | 0.767 | 3.891 | 0.187 | 1.72 | 0.63 | 4.75 | 0.292 | ||
| 67–100% | 18 | 14 | 77.8 | 3.65 | 1.789 | 7.444 | < 0.001** | 3.02 | 1.1 | 8.25 | 0.032* | ||
| Grade | |||||||||||||
| ≤1 | 19 | 7 | 36.8 | 1 | |||||||||
| 2 | 49 | 23 | 46.9 | 1.56 | 0.658 | 3.714 | 0.312 | ||||||
| 3 | 22 | 11 | 50.0 | 1.82 | 0.701 | 4.737 | 0.218 | ||||||
| Surgical margin (mm) | |||||||||||||
| >1 | 67 | 29 | 43.3 | 1 | |||||||||
| ≤1 | 23 | 12 | 52.2 | 1.19 | 0.607 | 2.332 | 0.613 | ||||||
| Lymphovascular invasion | |||||||||||||
| No | 19 | 6 | 31.6 | 1 | |||||||||
| Yes | 71 | 35 | 49.3 | 1.68 | 0.707 | 4.00 | 0.239 | ||||||
| Extracapsular extension | |||||||||||||
| No | 40 | 18 | 45.0 | 1 | |||||||||
| Yes | 50 | 23 | 46.0 | 0.98 | 0.528 | 1.819 | 0.949 | ||||||
| Tumor type | |||||||||||||
| ER/PR+ Her2− | 50 | 24 | 48.0 | ||||||||||
| ER/PR+ Her2+ | 20 | 8 | 40.0 | 0.83 | 0.369 | 1.846 | 0.641 | ||||||
| ER/PR− Her2+ | 9 | 3 | 33.3 | 0.69 | 0.205 | 2.282 | 0.537 | ||||||
| TNBC | 11 | 6 | 54.5 | 1.66 | 0.673 | 4.078 | 0.272 | ||||||
| Adjuvant chemotherapy or target therapy | |||||||||||||
| No | 51 | 22 | 43.1 | 1 | |||||||||
| Yes | 39 | 19 | 48.7 | 1.26 | 0.681 | 2.329 | 0.462 | ||||||
*, indicates P<0.05; **, indicates P<0.001; ***, indicates P close 0.05. LRR, loco-regional recurrence; HR, hazard ratio; BCS, breast conserving surgery; LN+ ratio, positive ratio of dissected lymph nodes; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor type2; TNBC, triple negative breast cancer.
All patients in this cohort who died had distant metastases. The risk factors affecting OS with statistical significance in the univariate analysis were the clinical N3 category (HR =10.73, P=0.042) and tumor type of TNBC (HR =4.09, P=0.006). However, only the tumor type TNBC (HR =3.04, P=0.048) showed statistical significance in the multivariate analysis (Table 4).
Table 4
| Characteristics | Total | Death | Univariate analysis | Multivariate analysis (adjusted) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | HR | 95% CI | P value | HR | 95% CI | P value | ||||||
| Lower | Upper | Lower | Upper | ||||||||||
| Age (years) | |||||||||||||
| ≤50 | 53 | 13 | 24.5 | 1 | |||||||||
| >50 | 37 | 10 | 27.0 | 1.10 | 0.73 | 1.66 | 0.652 | ||||||
| Surgery | |||||||||||||
| BCS | 24 | 5 | 20.8 | 1 | |||||||||
| Mastectomy | 66 | 18 | 27.3 | 1.45 | 0.54 | 3.90 | 0.466 | ||||||
| Clinical stage | |||||||||||||
| c stage II | 44 | 11 | 25.0 | 1 | |||||||||
| c stage III | 46 | 12 | 26.1 | 1.05 | 0.46 | 2.37 | 0.917 | ||||||
| Clinical T | |||||||||||||
| cT1–2 | 51 | 14 | 27.5 | 1 | |||||||||
| cT3 | 24 | 5 | 20.8 | 0.68 | 0.24 | 1.89 | 0.456 | ||||||
| cT4 | 15 | 4 | 26.7 | 0.96 | 0.32 | 2.91 | 0.937 | ||||||
| Clinical N | |||||||||||||
| cN0 | 11 | 1 | 9.1 | 1 | 1 | ||||||||
| cN1 micro/N1 | 57 | 14 | 24.6 | 3.14 | 0.41 | 23.99 | 0.271 | 2.90 | 0.38 | 22.51 | 0.308 | ||
| cN2 | 16 | 5 | 31.3 | 4.76 | 0.55 | 41.03 | 0.156 | 4.56 | 0.52 | 40.40 | 0.172 | ||
| cN3 | 6 | 3 | 50.0 | 10.73 | 1.09 | 106.14 | 0.042* | 5.83 | 0.53 | 64.69 | 0.151 | ||
| Response | |||||||||||||
| Partial | 52 | 14 | 26.9 | 1 | |||||||||
| Stable | 28 | 5 | 17.9 | 0.68 | 0.24 | 1.88 | 0.457 | ||||||
| Progression | 10 | 4 | 40.0 | 1.72 | 0.57 | 5.22 | 0.340 | ||||||
| Pathological T | |||||||||||||
| T1–2 | 10 | 4 | 40.0 | 1 | |||||||||
| T3 | 53 | 12 | 22.6 | 0.55 | 0.18 | 1.70 | 0.299 | ||||||
| T4 | 27 | 7 | 25.9 | 0.67 | 0.20 | 2.29 | 0.523 | ||||||
| Pathological N | |||||||||||||
| N0ihc/mi/1A | 51 | 11 | 21.6 | 1 | |||||||||
| N2a | 29 | 8 | 27.6 | 1.31 | 0.52 | 3.26 | 0.566 | ||||||
| N3a | 10 | 4 | 40.0 | 2.33 | 0.74 | 7.35 | 0.150 | ||||||
| Dissected lymph node | |||||||||||||
| <10# | 19 | 6 | 31.6 | 1 | |||||||||
| ≥10# | 71 | 17 | 23.9 | 0.60 | 0.24 | 1.53 | 0.283 | ||||||
| LN+ ratio | |||||||||||||
| 0–33% | 52 | 12 | 23.1 | 1 | |||||||||
| 34–66% | 20 | 3 | 15.0 | 0.78 | 0.22 | 2.79 | 0.707 | ||||||
| 67–100% | 18 | 8 | 44.4 | 2.27 | 0.93 | 5.56 | 0.073 | ||||||
| Grade | |||||||||||||
| ≤1 | 19 | 4 | 21.1 | 1 | |||||||||
| 2 | 49 | 13 | 26.5 | 1.39 | 0.45 | 4.25 | 0.570 | ||||||
| 3 | 22 | 6 | 27.3 | 1.63 | 0.46 | 5.80 | 0.449 | ||||||
| Surgical margin (mm) | |||||||||||||
| >1 | 67 | 18 | 26.9 | 1 | |||||||||
| ≤1 | 23 | 5 | 21.7 | 0.80 | 0.30 | 2.17 | 0.665 | ||||||
| Lymphovascular invasion | |||||||||||||
| No | 19 | 5 | 26.3 | 1 | |||||||||
| Yes | 71 | 18 | 25.4 | 1.05 | 0.39 | 2.82 | 0.929 | ||||||
| Extracapsular extension | |||||||||||||
| No | 40 | 9 | 22.5 | 1 | |||||||||
| Yes | 50 | 14 | 28.0 | 1.22 | 0.53 | 2.83 | 0.636 | ||||||
| Tumor type | |||||||||||||
| ER/PR+ Her2− | 50 | 12 | 24.0 | 1 | 1 | ||||||||
| ER/PR+ Her2+ | 20 | 4 | 20.0 | 0.85 | 0.28 | 2.65 | 0.785 | 0.72 | 0.23 | 2.27 | 0.569 | ||
| ER/PR− Her2+ | 9 | 1 | 11.1 | 0.46 | 0.06 | 3.57 | 0.461 | 0.49 | 0.06 | 3.78 | 0.49 | ||
| TNBC | 11 | 6 | 54.5 | 4.09 | 1.50 | 11.15 | 0.006* | 3.04 | 1.01 | 9.16 | 0.048* | ||
| Adjuvant chemotherapy or target therapy | |||||||||||||
| No | 51 | 11 | 21.6 | 1 | |||||||||
| Yes | 39 | 12 | 30.8 | 1.63 | 0.72 | 3.71 | 0.241 | ||||||
*, indicates P<0.05. LRR, loco-regional recurrence; HR, hazard ratio; BCS, breast conserving surgery; LN+ ratio, positive ratio of dissected lymph nodes; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor type2; TNBC, triple negative breast cancer.
The survival curves for LRR-free survival and RFS stratified by the therapeutic response after NAC, the positive lymph nodes ratio and the clinical N status compared by log-rank test showed statistical significance (P<0.05). The survival curves for RFS stratified by clinical N status showed a statistical trend (P=0.057) (Figure 1).
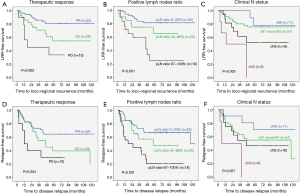
Discussion
Significant predictors of LRR for patients treated primarily with surgery are well documented in the literature. Among these predictors, the pathologic status of axillary lymph nodes, number of positive lymph nodes, and even adequacy of axillary dissection are shown to strongly predict LRR. However, the issues with these prognostic factors and the impact of regional nodal irradiation have rarely been addressed in patients primarily treated with NAC.
In this study, we aimed to examine the risk factors of LRR and relapse for patients receiving curative surgery following NAC to decide whether the level I axillary lymphatic region should be included in the target radiation field. Previous studies in non-NAC scenarios showed that dissection of at least 10 lymph nodes is adequate ALND (8-16), which is advantageous for disease control and OS (8-13,16).
There was no indication of radiotherapy for the level I axillary region in patients with adequate ALND because of significant treatment sequelae of a combination of ALND and axillary RT (17,18). Considering the serious side-effects of ALND, axillary RT gradually replaced ALND in clinical node-negative but pathologically SLNB positive patients (19).
Numbers of dissected lymph nodes
It is well known that the number of positive lymph nodes is a risk factor for disease control in pathologically node-positive patients after NAC (20,21). However, pathologically negative node after NAC may not indicate lack of residual tumor in the lymph node. Inadequate dissection or change in lymphoid tissue after NAC may also affect the pathological examination under the microscope.
In fact, there may be other reasons leading to inadequate axillary dissection after NAC. For example, it has been reported that the lymphoid tissue in lymph nodes changed after NAC, including lymphoid depletion, fibrosis and hyalinization (1,22-24). In addition, the shrinkage of axillary lymph nodes after NAC allows surgeons to identify the lymph nodes in difficult scenarios and lessen the dissected volume during surgery (24-26). Moreover, the pathological examination skills (such as blunt dissection or fat dissolving technique and serial sectioning or radiological evaluation) affect the accuracy and resolution of tumor cell assessment in lymph nodes. Furthermore, different therapeutic responses after NAC act as important predictive factors. For instance, the number of dissected lymph nodes of <10 nodes may indicate a good response. Therefore, dissection <10 nodes cannot be considered as inadequate ALND in a typical NAC scenario. In this study, although the difference in the number of dissected lymph nodes of more than or less than 10 nodes was statistically significant in univariate analysis for LRR, it was not a risk factor in multivariate analysis and overlapped with the positive ratio of dissected nodes. Hence, in patients treated with NAC, the dissected number of lymph nodes >10 cannot predict prognosis similar to that in patients receiving primary surgery followed by adjuvant chemoradiation (non-NAC). Further studies related to this characteristic are required for clarity.
Positive lymph nodes ratio
Several studies in the past reported a positive lymph nodes ratio as a prognostic factor in patients treated primarily with surgery (non-NAC) (27-30). The positive lymph nodes ratio may be a better prognostic factor than the dissected lymph nodes >10 nodes in patients with positive axillary nodes after NAC. Chen et al. reported that the positive lymph nodes ratio affects RFS and OS in patients with positive pathological lymph nodes after NAC. Moreover, they also found that, in patients with negative pathological lymph nodes after NAC, the total number of dissected lymph nodes (<10 nodes) affected RFS and OS (31). Wu et al. also revealed positive lymph nodes ratio as an independent prognostic factor of loco-regional control, distant metastasis (DM)-free survival, disease-free survival (DFS) and OS; however, ypN stage had no effect on prognosis (32). In the study by Cho et al., it was reported that the positive lymph nodes ratio is significantly associated with DFS and OS. However, the pathologic N stage was not significantly associated with DFS and OS (33).
In this study, we also found that the positive lymph nodes ratio was associated with loco-regional control and relapse in axillary node-positive patients after NAC. Patients with 67–100% lymph nodes positive ratio showed greater LRR risk and relapse (HR =4.32 and P=0.025 and HR =3.02 and P=0.032, respectively). Thus, positive lymph nodes ratio may have more clinical significance than pathological lymph node status in patients receiving NAC.
Axillary recurrence
In patients receiving surgery primarily, a low proportion of patients in the early-stage showed axillary recurrence (0.2–2%) (19,34,35). A high rate of axillary recurrence (0.3–7%) was recorded only in early stage patients with positive lymph nodes identified by SLNB without further axillary treatment (36). In a 10-year report of NSABP B-18 and B-27 trials, patients treated with NAC and lumpectomy or mastectomy showed regional recurrence of 0–8.7% according to their therapeutic response (1). This study presented 90 patients with clinical stage II and III breast cancer with positive axillary lymph nodes after NAC, of which 26 patients had LRR and only 9 patients had axillary recurrence. Of the 9 patients with axillary recurrence, only 3 patients received irradiation to axillary level I region. Only few events were available to analyze the risks of axillary recurrence, we have presented the characteristics of patients with axillary recurrence in Table 5. There was obviously a trend that patients with primary tumor in the upper-outer quadrant and those with extracapsular extension of lymph nodes tended to have axillary recurrence. In addition, 6 of 9 patients had lymph nodes positive ratio >50%. LRR rate was higher in our study than that shown in NSABP B18 and B27 (LRR: 0–8.7%), which was possible only because we included patients with positive lymph nodes after NAC [NSABP analysis data including negative axillary lymph nodes (ypN0) after NAC]. In the NSABP studies, no patients with clinical N2/N3 status (stage III) and >50% patients with cT1–2N0 (stage IA/IIA) in NSABP B18 (65%) and B27 (51%) were recorded. Only patients with clinical stages II and III breast cancer were enrolled in this study and this population presented with higher risks of LRR.
Table 5
| Case No. | Age at diagnosis | Operation method | Clinical T/N | Pathological T/N | Response after NAC | LN+ ratio group | LN+ ratio | Number of dissected lymph nodes | Tumor location | ECE | Level I RT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 59 | Total mastectomy | cT2N1 | ypT2N3a | SD | 67–100% | 68.8% | 16 | Upper and LOQ§ | + | – |
| 2 | 56 | Total mastectomy | cT2N1 | ypT1aN3a | PR | 67–100% | 100.0% | 11 | UOQ | + | + |
| 3 | 57 | BCS | cT1cN2 | ypT1cN1mi | SD | 67–100% | 75.0% | 4 | UIQ | – | – |
| 4 | 49 | Total mastectomy | cT3N1 | ypT3N3a | SD | 67–100% | 100.0% | 13 | UOQ | – | – |
| 5 | 57 | BCS | cT2N2 | ypT1cN1a | PR | 34–66% | 50.0% | 4 | Central | + | + |
| 6 | 35 | Total mastectomy | cT4bN3 | ypT4bN2a | SD | 34–66% | 46.7% | 15 | UOQ | + | – |
| 7 | 47 | BCS | cT2N1 | ypT2N2a | SD | 34–66% | 60.0% | 10 | UOQ | – | – |
| 8 | 66 | Total mastectomy | cT2N1 | ypT2N1a | PD | 34–66% | 42.9% | 7 | UIQ | – | + |
| 9 | 54 | Total mastectomy | cT2N1 | ypT3N1a | PD | 1–33% | 11.1% | 9 | UOQ | + | – |
Characteristics of 9 patients with axillary lymph nodes recurrence in this cohort study showed 6 (66.7%) patients had positive ratio of dissected lymph nodes >50%, 6 (66.7%) patients had primary tumor at upper outer quadrant or 12 o’clock and 5 (55.6%) patients had lymph nodes metastasis with extracapsular extension. §, two separate tumors at 2 cm from areolar border at 12 o’clock and 3 cm from areolar border at 8 o’clock. BCS, breast conserving surgery; NAC, neoadjuvant chemotherapy; LN+, lymph node positive; ECE, extracapsular extension; RT, radiotherapy; PR, partial response; SD, stable disease; PD, progressive disease; UIQ, upper inner quadrant; UOQ, upper outer quadrant; LOQ, lower outer quadrant.
In non-NAC situations, the study by Kaygusuz et al. revealed the presence of extracapsular extension indicates more advanced axillary disease and is associated with higher DM rate (37). Another study showed the presence of extracapsular extension in association with higher recurrence and mortality rate (38) and the perpendicular diameter of extra-nodal growth (>3 mm) affected DFS and cancer-specific survival (39). In patients treated using NAC, the presence of extracapsular extension could also predict the DFS. There was no evidence showing that the presence of extracapsular extension can contribute to regional recurrence. However, the axillary recurrence in this study seems to be associated with the presence of extracapsular extension and tumor located in the upper-outer quadrant. Further studies are required to investigate these factors to design the optimal radiation treatment field, particularly for those patients with only SLNB or inadequate ALND.
Biomarker/molecular type
According to the MSKCC retrospective data (40), patients with TNBC showed the highest LRR after NAC, mastectomy, and PMRT; TNBC patients with residual disease after NAC showed greater risk for LRR than patients with pCR. However, this study included only 11 (12%) patients with TNBC, which made correlation analysis difficult. Assessment of the molecular type was an accurate predictor of survival in patients treated with NAC (41-44). Patients with HER2 positive showed good response to NAC with target therapy and easily achieved pCR. Of the common molecule types, the prognosis of patients with HER2 positive type was most related to pCR (6).
In this study, 2 of 9 patients (22.2%) had ER(−)/PR(−)/HER2(+) and 4 of 11 TNBC patients (36.4%) had LRR; only 3 of 9 patients (33.3%) with ER(−)/PR(−)/HER2(+) and 6 of 11 patients with TNBC (54.5%) had DM. Although the limited number cases could not show statistical significance, Herceptin-application may improve disease control in patients with HER2 positive results, as shown in patients with ER(−)/PR(−)/HER2(+) and TNBC in this study. However, we only found that the TNBC type showed statistical significance in the uni-/multi-variate analysis for OS, which could be due to the small number of enrolled patients and the relatively short-term follow-up duration.
This study included patients with pathologically positive lymph nodes. Exclusion of patients with pCR (ypTis/T0 N0) decreased the strong impact of therapeutic response on other risk factors independent of achieving statistical significance. Univariate and multivariate analyses revealed that therapeutic response, clinical N status, and positive lymph nodes ratio were risk factors of LRR and DM. Several studies have showed therapeutic response (41-43), clinical N status (45,46) and positive ratio of dissected LN (31-33) as predictors of disease control. However, the risk factors found by these studies, such as grade 3 (47), presence of lymphovascular invasion (45-47), ypN2–3 (47), primary tumor size (45-47), pathologic tumor size after NAC (46), age (1), molecular type (TNBC) (6,40), and positive surgical margin (48) failed to achieve any statistical significance or trends as shown in other studies. It may be attributed to the relatively fewer number of cases and events, short follow-up period, and selection bias which are the inherent features of a retrospective study.
The pathological lymph nodes status, such as ypN1 and ypN2 in NAC scenarios was not obviously significant in predicting disease control as in non-NAC scenarios, both in the present study and others NAC studies. This prognostic factor may be interfered by NAC. Whether positive lymph node ratio is a better prognostic factor than only the number of positive lymph nodes requires further detailed investigation.
In non-NAC scenarios, the number of positive axillary lymph nodes and dissected lymph nodes can be used to determine the loco-regional treatment such as radiotherapy indication and field-design (axillary irradiation) after primary surgery. However, in NAC scenarios, the predictive value of both the factors would be affected by several reasons such as therapeutic response after NAC, lymphoid depletion in axillary nodes after NAC, decreasing dissected volume of ALND, and pathological techniques. In this study, positive lymph nodes ratio seemed to be more valuable for prognosis than the number of positive axillary lymph nodes and dissected lymph nodes. Despite insufficient evidence, irradiation to axillary level I region could be considered when ypN+ patients present with high positive ratio and low number of dissected lymph nodes.
This study has some limitations. First, it is a retrospective study and therefore includes some bias. Longer follow-up period and a larger sample size in the future studies will help provide better result for breast cancer study. In response, the Alliance 011202 trial phase-III randomized trial, including clinical T1–3N1M0 patients with positive axillary lymph nodes after NAC, has been initiated to address the question about axillary recurrent risks and their management.
Conclusions
The consensus on the exact role of radiotherapy in the management of patients with breast cancer undergoing NAC with regard to the therapeutic response remains unclear. In this study, we recorded poor therapeutic response, advanced clinically positive lymph nodes, and higher proportional positivity of dissected lymph nodes showing poor outcome regarding to the loco-regional control and RFS among patients with positive axillary lymph nodes after NAC. The decision whether radiotherapy can be delivered to the regional lymphatic areas, including axilla, should depend on the chemotherapeutic response and surgical extent, particularly in patients with incomplete response of the lymph nodes with high positive lymph nodes ratio. Future prospective studies will provide us with more accurate risk predictors of LRR for better planning of the treatment design.
Acknowledgments
Language editing assistance: Enago for the English language review.
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Breast Cancer Multidiscipline Group of Changhua Christian Hospital (CCH) and the ethic approval was obtained from the committee on human experimentation of the same institution (CCH IRB No. 180313). Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mamounas EP, Anderson SJ, Dignam JJ, et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol 2012;30:3960. [Crossref] [PubMed]
- Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 1997;15:2483-93. [Crossref] [PubMed]
- van der Hage JA, van de Velde CJ, Julien JP, et al. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol 2001;19:4224-37. [Crossref] [PubMed]
- Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol 1999;17:460-9. [Crossref] [PubMed]
- Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol 2008;26:778-85. [Crossref] [PubMed]
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384:164-72. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Salama JK, Heimann R, Lin F, et al. Does the number of lymph nodes examined in patients with lymph node-negative breast carcinoma have prognostic significance? Cancer 2005;103:664-71. [Crossref] [PubMed]
- Sosa JA, Diener-West M, Gusev Y, et al. Association between extent of axillary lymph node dissection and survival in patients with stage I breast cancer. Ann Surg Oncol 1998;5:140-9. [Crossref] [PubMed]
- Mersin H, Yıldırım E, Bulut H, et al. The prognostic significance of total lymph node number in patients with axillary lymph node-negative breast cancer. Eur J Surg Oncol 2003;29:132-8. [Crossref] [PubMed]
- Polednak AP. Survival of lymph node-negative breast cancer patients in relation to number of lymph nodes examined. Ann Surg 2003;237:163. [Crossref] [PubMed]
- Weir L, Speers C, D’yachkova Y, et al. Prognostic significance of the number of axillary lymph nodes removed in patients with node-negative breast cancer. J Clin Oncol 2002;20:1793-9. [Crossref] [PubMed]
- Van der Wal B, Butzelaar R, Van der Meij S, et al. Axillary lymph node ratio and total number of removed lymph nodes: predictors of survival in stage I and II breast cancer. Eur J Surg Oncol 2002;28:481-9. [Crossref] [PubMed]
- Somner J, Dixon J, Thomas J. Node retrieval in axillary lymph node dissections: recommendations for minimum numbers to be confident about node negative status. J Clin Pathol 2004;57:845-8. [Crossref] [PubMed]
- Kiricuta CI, Tausch J. A mathematical model of axillary lymph node involvement based on 1446 complete axillary dissections in patients with breast carcinoma. Cancer 1992;69:2496-501. [Crossref] [PubMed]
- Joslyn SA, Konety BR. Effect of axillary lymphadenectomy on breast carcinoma survival. Breast Cancer Res Treat 2005;91:11-8. [Crossref] [PubMed]
- Wazer DE, Erban JK, Robert NJ, et al. Breast conservation in elderly women for clinically negative axillary lymph nodes without axillary dissection. Cancer 1994;74:878-83. [Crossref] [PubMed]
- Liljegren G, Holmberg L. Arm morbidity after sector resection and axillary dissection with or without postoperative radiotherapy in breast cancer stage I. Results from a randomised trial. Eur J Cancer 1997;33:193-9. [Crossref] [PubMed]
- Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol 2014;15:1303-10. [Crossref] [PubMed]
- Colleoni M, Bagnardi V, Rotmensz N, et al. A risk score to predict disease-free survival in patients not achieving a pathological complete remission after preoperative chemotherapy for breast cancer. Ann Oncol 2009;20:1178-84. [Crossref] [PubMed]
- Meric F, Mirza NQ, Buzdar AU, et al. Prognostic implications of pathological lymph node status after preoperative chemotherapy for operable T3N0M0 breast cancer. Ann Surg Oncol 2000;7:435-40. [Crossref] [PubMed]
- Erbes T, Orlowska-Volk M, Zur Hausen A, et al. Neoadjuvant chemotherapy in breast cancer significantly reduces number of yielded lymph nodes by axillary dissection. BMC Cancer 2014;14:4. [Crossref] [PubMed]
- Neuman H, Carey LA, Ollila DW, et al. Axillary lymph node count is lower after neoadjuvant chemotherapy. Am J Surg 2006;191:827-9. [Crossref] [PubMed]
- Newman LA, Pernick NL, Adsay V, et al. Histopathologic evidence of tumor regression in the axillary lymph nodes of patients treated with preoperative chemotherapy correlates with breast cancer outcome. Ann Surg Oncol 2003;10:734-9. [Crossref] [PubMed]
- Bélanger J, Soucy G, Sidéris L, et al. Neoadjuvant chemotherapy in invasive breast cancer results in a lower axillary lymph node count. J Am Coll Surg 2008;206:704-8. [Crossref] [PubMed]
- Kuroi K, Toi M, Tsuda H, et al. Issues in the assessment of the pathologic effect of primary systemic therapy for breast cancer. Breast Cancer 2006;13:38-48. [Crossref] [PubMed]
- Vinh-Hung V, Verkooijen HM, Fioretta G, et al. Lymph node ratio as an alternative to pN staging in node-positive breast cancer. J Clin Oncol 2009;27:1062-8. [Crossref] [PubMed]
- Danko ME, Bennett KM, Zhai J, et al. Improved staging in node-positive breast cancer patients using lymph node ratio: results in 1,788 patients with long-term follow-up. J Am Coll Surg 2010;210:797-805. e1.
- Chagpar AB, Camp RL, Rimm DL. Lymph node ratio should be considered for incorporation into staging for breast cancer. Ann Surg Oncol 2011;18:3143. [Crossref] [PubMed]
- Ahn SH, Kim HJ, Lee JW, et al. Lymph node ratio and pN staging in patients with node-positive breast cancer: a report from the Korean breast cancer society. Breast Cancer Res Treat 2011;130:507. [Crossref] [PubMed]
- Chen S, Liu Y, Huang L, et al. Lymph node counts and ratio in axillary dissections following neoadjuvant chemotherapy for breast cancer: a better alternative to traditional pN staging. Ann Surg Oncol 2014;21:42-50. [Crossref] [PubMed]
- Wu SG, Li Q, Zhou J, et al. Using the lymph node ratio to evaluate the prognosis of stage II/III breast cancer patients who received neoadjuvant chemotherapy and mastectomy. Cancer Res Treat 2015;47:757. [Crossref] [PubMed]
- Cho DH, Bae SY, You JY, et al. Lymph node ratio as an alternative to pN staging for predicting prognosis after neoadjuvant chemotherapy in breast cancer. Kaohsiung J Med Sci 2018;34:341-7. [Crossref] [PubMed]
- Volders J, van la Parra R, Bavelaar-Croon C, et al. Discordance between number of scintigraphic and perioperatively identified sentinel lymph nodes and axillary tumour recurrence. Breast 2014;23:159-64. [Crossref] [PubMed]
- Andersson Y, de Boniface J, Jönsson PE, et al. Axillary recurrence rate 5 years after negative sentinel node biopsy for breast cancer. Br J Surg 2012;99:226-31. [Crossref] [PubMed]
- Francissen CM, Dings PJ, van Dalen T, et al. Axillary recurrence after a tumor-positive sentinel lymph node biopsy without axillary treatment: a review of the literature. Ann Surg Oncol 2012;19:4140-9. [Crossref] [PubMed]
- Kaygusuz EI, Cetiner H, Yavuz H. Clinico-pathological significance of extra-nodal spread in special types of breast cancer. Cancer Biol Med 2014;11:116. [PubMed]
- Nottegar A, Veronese N, Senthil M, et al. Extra-nodal extension of sentinel lymph node metastasis is a marker of poor prognosis in breast cancer patients: A systematic review and an exploratory meta-analysis. Eur J Surg Oncol 2016;42:919-25. [Crossref] [PubMed]
- Aziz S, Wik E, Knutsvik G, et al. Extra-nodal extension is a significant prognostic factor in lymph node positive breast cancer. PLoS One 2017;12:e0171853 [Crossref] [PubMed]
- Yang TJ, Morrow M, Modi S, et al. The effect of molecular subtype and residual disease on locoregional recurrence in breast cancer patients treated with neoadjuvant chemotherapy and postmastectomy radiation. Ann Surg Oncol 2015;22:495-501. [Crossref] [PubMed]
- Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 2008;26:1275-81. [Crossref] [PubMed]
- von Minckwitz G, Untch M, Blohmer J-U, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012;30:1796-804. [Crossref] [PubMed]
- Bonnefoi H, Litière S, Piccart M, et al. Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: a landmark and two-step approach analyses from the EORTC 10994/BIG 1-00 phase III trial. Ann Oncol 2014;25:1128-36. [Crossref] [PubMed]
- Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 2007;25:4414-22. [Crossref] [PubMed]
- Chen AM, Meric-Bernstam F, Hunt KK, et al. Breast conservation after neoadjuvant chemotherapy. Cancer 2005;103:689-95. [Crossref] [PubMed]
- Akay CL, Meric-Bernstam F, Hunt KK, et al. Evaluation of the MD Anderson Prognostic Index for local-regional recurrence after breast conserving therapy in patients receiving neoadjuvant chemotherapy. Ann Surg Oncol 2012;19:901-7. [Crossref] [PubMed]
- Matsuda N, Hayashi N, Ohde S, et al. A nomogram for predicting locoregional recurrence in primary breast cancer patients who received breast-conserving surgery after neoadjuvant chemotherapy. J Surg Oncol 2014;109:764-9. [Crossref] [PubMed]
- Beriwal S, Schwartz GF, Komarnicky L, et al. Breast-Conserving Therapy after Neoadjuvant Chemotherapy: Long-term Results. Breast J 2006;12:159-64. [Crossref] [PubMed]
Cite this article as: Yang SJ, Pi CP, Chang TH, Liu MT, Huang CC, Hung LC, Chou TW, Lin JB, Wang SH. Prognostic factors of axillary lymph node-positive patients in clinical stage II and III breast cancer after neoadjuvant chemotherapy. Ther Radiol Oncol 2018;2:37.


