
Establishment of treatment strategy and preliminary outcome for synchronous head and neck and thoracic esophageal squamous cell carcinoma
Introduction
Head and neck cancer and esophageal cancer possess high incidence and mortality rate worldwide, especially in Asian countries. Among these cancers, approximately 90% are squamous cell carcinomas (SCCs). They share similar risk factors, including cigarette smoking, alcohol drinking, and upper gastrointestinal (UGI) cancer history (1). The incidence of synchronous head and neck SCC (HNSCC) and esophageal SCC (ESCC) is approximately 12–24% (2-4).
For patients with HNSCC, many strategies are utilized such as surgery, definitive radiotherapy (RT), and chemoradiation (CCRT). HNSCC is relatively sensitive to radiation and anticancer drugs such as cisplatin. Therefore, CCRT is increasingly used not only in definitive settings but also as sequential treatment after induction chemotherapy of hypopharyngeal cancer, especially in locally advanced disease where organ preservation and lower rates of complications and mortality compared to surgery can be achieved. The radiation dose is typically 66–70 Gy in high risk planning target volume (PTVH), 54–63 Gy in low and intermediate risk PTV (PTVL and PTVM). Gross tumor volume (GTV) consists of primary tumor and involved lymph nodes. High risk clinical tumor volume (CTVH) is defines as GTV plus margins as least 5 mm. Intermediate risk CTV (CTVM) includes lymph nodes area of high risk subclinical disease. Bilateral neck especially ipsilateral neck is often treated as CTVM, which depends on clinical stage, primary tumor sites and involved lymph nodes. Low risk CTV (CTVL) is defined as lymph nodes area of low risk disease.
Neoadjuvant CCRT, definitive CCRT, and esophagectomy are acceptable treatment options for patients with ESCC. The treatment method using neoadjuvant CCRT followed by surgery is the most common approach in both resectable and unresectable ESCC setting. The radiation dose is suggested with 41.4–50.4 Gy in 1.8–2.0 Gy per day (5,6). GTV is defined as primary tumor and involved lymph nodes. CTVH is defined as GTV plus nearby regions at high risk of microscopic disease. CTVL is defined as primary tumor plus 3–4 cm expansion superiorly and inferiorly with a 1 cm radial expansion. Elective treatment of node-bearing regions is added in CTVL depending on the location of primary tumors.
Advances in imaging technologies, such as computed tomography and magnetic resonance imaging have enabled the possibility of transitioning from two-dimensional treatment to three-dimensional conformal RT. After the introduction of inverse planning, the use of intensity-modulated radiation therapy (IMRT) with non-uniform beam intensities grew rapidly, resulting in a more conformal dose distribution. Now, IMRT is the standard practice for many tumors. Moreover, IMRT with simultaneous integrated boost (SIB) has proved to be a feasible treatment option in HNSCC, increasing total delivered dose in a shortened treatment time with the result of increased tumor control (7).
For treatment of synchronous HNSCC and ESCC, the optimal combination of therapeutic modalities remains controversial with limited clinical reports (8-10). Shinoto et al. reported treatment outcome by simultaneous CCRT with acceptable results and other investigators demonstrated poor outcomes (11,12). In comparison to HNSCC, the prognosis of ESCC without radical surgery is poorer with a 5-year survival rate less than 25% (5). To downstage the ESCC for surgery and avoid treatment delay of HNSCC, the design of multidisciplinary and unique treatment strategy for simultaneous curative therapy is critical. This comprehensive treatment design should consist of considerations for RT technique, chemotherapeutics, toxicity and putative surgical procedures.
For synchronous HNSCC and ESCC, we created a multidisciplinary team to tailor patient’s treatment strategy. In the present report, we introduced a strategy of simultaneous CCRT with 2-step SIB technique followed by evaluation of surgery for esophageal lesions.
Methods
Design of treatment
Multidisciplinary team work
All patients were included in multidisciplinary model, and all decisions were made by patients after discussing with experienced GI doctors, surgeons, medical oncologists and radiation oncologists.
Development of treatment guideline for synchronous HNSCC and ESCC
After extensive discussion, the treatment guideline for synchronous HNSCC and ESCC in our institution was established and listed as flow chart, which was reviewed every year. The flow chart was demonstrated in Figure 1.
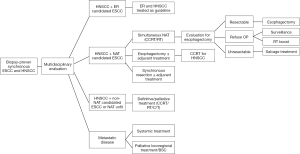
Patients
All patients had biopsy-proven clinical stage I–III HNSCC and stage I–III ESCC at the same time without previous history of chemotherapy or irradiation. The patients were required to have a World Health Organization performance status of 0 to 2. Metastatic disease was excluded. All patients were treated with curative intent.
Twelve patients with synchronous HNSCC and ESCC who received treatment between January 2014 and July 2017 at our institution were retrospectively reviewed. Six patients were considered neoadjuvant therapy (NAT) candidates after multidisciplinary team discussion. Another 5 patients refused esophagectomy were treated by definitive simultaneous RT or CCRT. One patient received esophagectomy followed by sequential treatment of simultaneous CCRT.
Pre-treatment evaluation included a complete medical history, physical examination, complete blood count and biochemistry survey, chest radiography, head and neck CT or MRI, chest and abdominal CT, ENT endoscopy with biopsy, upper GI endoscopy with endoscopic ultrasonography and biopsy, and whole-body positron emission tomography (PET) scan. The tumor stages were determined according to the 7th American Joint Committee on Cancer (AJCC) staging system.
This study was approved by the Institutional Review Board in our institution.
CCRT
All patients underwent CT simulation in supine position and were immobilized with thermoplastic mask for head and neck and alpha cradle for chest region. Planning CT images with a maximum slice thickness of 3 mm were acquired through the entire head and neck, thorax and upper abdomen. The GTV consisted of the primary tumor and involved lymph nodes, using imaging studies that included CT, endoscopic ultrasonography and PET scan. CTVH was considered to be at significant risk of microscopic disease, and defined as GTV plus a margin which stratified as above according to RTOG atlas for delineation. Elective nodal irradiation was included in CTVM and CTVL. PTV was defined as CTV plus a 0.3-cm margin in all directions for HNSCC, and 0.5–0.7 cm margin in all directions for ESCC.
Treatment plans were generated using 6- or 10-MV photons. Eleven patients were treated with linear accelerators (Eclipse Treatment Planning System; Varian Medical Systems Inc., Palo Alto, CA), and 1 patient was treated with helical TomoTherapy (HT) (TomoTherapy, Madison, WI, USA). In NAT candidate, all patients were treated by simultaneous CCRT with IMRT with 2-step SIB technique. The prescribed doses for HNSCC were 70 Gy for PTVH (2 Gy/fraction), 63 Gy for PTVM (1.8 Gy/fraction), 56 Gy for PTVL (1.6 Gy/fraction). The prescribed doses for ESCC were 48 Gy for PTVH (2 Gy/fraction), 43.2 Gy for PTVL (1.8 Gy/fraction). There were 24 fractions in step 1. PTVH was covered with 2 Gy per fraction, PTVM of HNSCC and PTVL of ESCC were covered with 1.8 Gy per fraction, and PTVL of HNSCC was covered with 1.6 Gy per fraction. Then re-simulation was arranged for step 2 to treat HNSCC to prescribed dose. There were 11 fractions in step 2 with SIB technique on PTVH, PTVM and PTVL of HNSCC. The goals were to deliver the prescribed dose to ≥95% of the PTV and deliver 95% of the prescribed dose to ≥99% of the PTV. Dose distribution was shown in Figure 2.
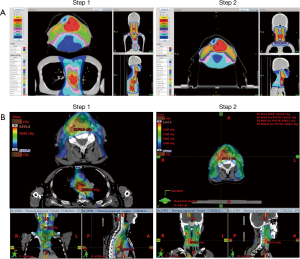
The RT dose of lower neck especially left supraclavicular fossa was reduced from 56 Gy in 35 fractions to 43.2 Gy in 24 fractions to prevent complications at anastomosis site.
In non-NAT candidate, 2 patients were treated with 2-step SIB technique and 3 patients with 3-step strategy. The radiation dose of ESCC tumor bed and periesophageal lymph nodes area was 48–50.4 Gy. HNSCC tumor bed and lymph nodes were covered by 70 Gy, with high risk lymph nodes area to 63 Gy, and subclinical lymph nodes area to 50–56 Gy.
Another one patient received esophagectomy first and sequential treatment with simultaneous CCRT with 2-step SIB technique. The radiation dose to periesophageal lymph nodes area was 50.4 Gy in 28 fractions, HNSCC tumor bed was 70 Gy in 35 fractions, and high risk lymph nodes area was to 63 Gy in 35 fractions.
Nine patients were treated with weekly cisplatin (30 mg/m2) (13-15), 2 patients with carboplatin for poor renal function, and 1 patient refused chemotherapy. In NAT candidate, all patients were treated with weekly cisplatin (30 mg/m2) over four courses, which was delivered by i.v. infusion.
Response assessment and toxicity evaluation
After CCRT, head and neck, chest and abdominal CT, ENT and upper GI endoscopy, PET scan was arranged for response assessment. If tumor is considered resectable in NAT candidate after assessment, esophagectomy for esophageal lesion was suggested and performed by experienced surgeon.
Hemoglobin (Hgb) and platelet (Plt) counts, WBCs and ANCs were collected weekly (1 week before Day 1 of treatment to the week after CCRT for WBCs and ANCs, or 90 days after CCRT for Hgb and Plt counts). These values were scored by the Common Terminology Criteria for Adverse Events (v. 4.0) with the highest grade of toxicity. Radiation-related pneumonitis, dermatitis, mucositis, esophageal stenosis and fistula were also recorded.
Operation
All esophagectomy was done under thoracoscopic guide after confirming no metastasis. The esophagus was identified, looped, divided and resected. Gastric tube was created for reconstruction. Then the gastric tube was pull from the hiatus to the neck, and the anastomosis was done to proximal esophagus and fixed to the anterior cervical ligament. The paraesophageal space was dissected from the thoracic inlet to the hiatus. The lymph nodes around the tumor and mediastinum were also dissected.
Results
Patient characteristics
A summary of the baseline characteristics of the 12 patients is listed in Table 1. The median age at diagnosis was 57 years old (range, 46–63 years old). The median follow-up duration was 10.0 months (range, 7.2–25.8 months).
Table 1
| Characteristics | Number (%) |
|---|---|
| Age (y) | 57 (range, 46–63) |
| Gender | |
| Male | 11 (91.7) |
| Female | 1 (8.3) |
| Pre-treatment BMI | |
| Median | 19.5 |
| Range | 13.7–23.8 |
| Post-treatment BMI | |
| Median | 18.7 |
| Range | 12.5–23.7 |
| Cigarette smoking | |
| Yes | 7 (58.3) |
| No | 5 (41.7) |
| Alcohol drinking | |
| Yes | 8 (66.7) |
| No | 4 (33.3) |
| ESCC stage | |
| I | NAT: 1 (16.7)/non-NAT: 0 (0)/Adj: 0 (0) |
| II | NAT: 2 (33.3)/non-NAT: 1 (20.0)/Adj: 1 (100.0) |
| III | NAT: 3 (50.0)/non-NAT: 4 (80.0)/Adj: 0 (0) |
| IV | NAT: 0 (0)/non-NAT: 0 (0)/Adj: 0 (0) |
| ESCC location | |
| Upper | NAT: 0 (0)/non-NAT: 1 (20.0)/Adj: 0 (0) |
| Middle | NAT: 2 (33.3)/non-NAT: 3 (60.0)/Adj: 1 (100.0) |
| Lower | NAT: 4 (66.7)/non-NAT: 1 (20.0)/Adj: 0 (0) |
| HNSCC stage | |
| I | NAT: 2 (33.3)/non-NAT: 1 (20.0)/Adj: 0 (0) |
| II | NAT: 2 (33.3)/non-NAT: 1 (20.0)/Adj: 0 (0) |
| III | NAT: 2 (33.3)/non-NAT: 1 (20.0)/Adj: 0 (0) |
| IV | NAT: 0 (0)/non-NAT: 2 (40.0)/Adj: 1 (100.0) |
| HNSCC location | |
| Oropharynx | NAT: 1 (16.7)/non-NAT: 2 (40.0)/Adj: 0 (0) |
| Hypopharynx | NAT: 5 (83.3)/non-NAT: 3 (60.0)/Adj: 1 (100.0) |
HNSCC, head and neck squamous cell carcinoma; ESCC, esophageal squamous cell carcinoma; NAT, neoadjuvant therapy candidate; Adj, adjuvant CCRT.
Treatment compliance and outcomes
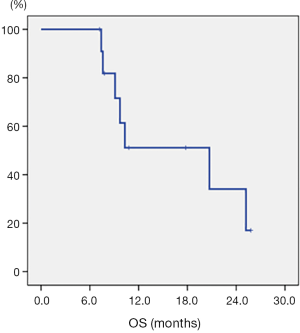
All patients completed the RT without dose and field reduction. The 1- and 2-year OS of the 12 patients with synchronous HNSCC and ESCC were 51.1% and 34.1%, respectively (Figure 3). In NAT candidate, one patient held RT for one week for severe mucositis, and all patients were treated with weekly cisplatin (30 mg/m2) without dose reduction. There were no treatment-related deaths. Clinical complete response (cCR) was noted in 3 patients with SUVmax of PET/CT scan reduced to lower than 3 or undetectable with formal reports after CCRT. Two patients received esophagectomy with pCR, and 1 patient received pan-endoscopy with negative findings in esophageal biopsy at primary lesions. Two patients refused surgery after CCRT for personal reason, and another one refused for cCR after CCRT. Surgery was performed in 3 patients. The median time between the end of CCRT and surgery was 2.1 weeks (range, 1.0–2.3 weeks). Pathological complete response (pCR) was reported in these 3 patients without residual carcinoma. Two patients with operation developed locoregional recurrence (LRR) and distant metastasis (DM). All recurrences were from periesophageal lymph nodes, including one in non-OP group. No recurrence was reported in head and neck field. One patient with surgery died of sepsis with pneumonia after 9.1 months from CCRT. One patient without surgery developed LRR and DM after 2.5 months. This patient died of empyema after 10.3 months from CCRT.
In non-NAT candidate, cCR was noted in 1 patient with PET/CT scan after CCRT. Esophageal biopsy reported acute inflammation. Four patients developed in-field LRR in esophageal tumor bed or periesophageal lymph nodes. Three patients developed DM. Four patients died after median 15.2 months (range, 7.4–25.2 months) from CCRT. In adjuvant CCRT, patient died of pneumonia after 7.6 months from CCRT without LRR and DM. Treatment outcomes were summarized in Table 2.
Table 2
| Patients | HNSCC stage | ESCC stage | Day to LRR (months) | Day to DM (months) | Survival |
|---|---|---|---|---|---|
| NAT candidate: OP | |||||
| 1 | I | IB | – | – | Alive |
| 2 | III | IIIB | 6.2 | 6.2 | Died |
| 3 | II | IIB | 17.7 | 17.7 | Lost |
| NAT candidate: non-OP | |||||
| 1 | III | IIA | – | – | Alive |
| 2 | I | IIIA | 2.5 | 2.5 | Died |
| 3 | II | IIIA | – | – | Alive |
| Non-NAT candidate | |||||
| 1 | IVA | IIIA | 3.2 | 6.5 | Lost |
| 2 | II | IIA | 7.3 | 7.3 | Died |
| 3 | IVA | IIIB | – | – | Died |
| 4 | I | IIIC | 2.8 | 2.8 | Died |
| 5 | III | IIIA | 6.4 | – | Died |
| Adjuvant CCRT | |||||
| 1 | IVA | IIB | – | 6.4 | Died |
oHNSCC, head and neck squamous cell carcinoma; ESCC, esophageal squamous cell carcinoma; NAT, neoadjuvant therapy; LRR, locoregional recurrence; DM, distant metastasis.
Adverse events
In NAT candidate, 4 patients developed grade 3 neutropenia. Two patients developed grade 3 anemia, and treated by blood transfusion. Two patients developed grade 3 esophageal stenosis. One patient was cared with feeding jejunostomy, and another one with esophageal stent. Two patients developed grade 3 mucositis. No grade 3 dermatitis was observed. Radiation pneumonitis was almost asymptomatic with grade 2 toxicity in 1 patient, treated by oral medication and recovered soon.
In non-NAT candidate, 2 patient developed grade 3 neutropenia. Three patients developed grade 3 anemia, and treated by blood transfusion. Three patients developed grade 3 esophageal stenosis. One patient was cared with feeding jejunostomy, one with esophageal stent, and another one with repeated esophageal dilation. No grade 3 mucositis and dermatitis was observed. Radiation pneumonitis was all asymptomatic in these 5 patients. Toxicities profile was recorded in Table 3.
Table 3
| Toxicity | CTCAE grade | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Toxicity of NAT candidate | |||||
| Leukopenia | 0 | 0 | 2 | 4 | 0 |
| Neutropenia | 1 | 0 | 1 | 4 | 0 |
| Anemia | 0 | 0 | 4 | 2 | 0 |
| Thrombocytopenia | 0 | 3 | 0 | 3 | 0 |
| Esophageal stenosis | 4 | 0 | 0 | 2 | 0 |
| Esophageal fistula | 6 | 0 | 0 | 0 | 0 |
| Mucositis | 0 | 2 | 2 | 2 | 0 |
| Dermatitis | 0 | 2 | 4 | 0 | 0 |
| Pneumonitis | 0 | 5 | 1 | 0 | 0 |
| Toxicity of non-NAT candidate | |||||
| Leukopenia | 0 | 1 | 2 | 2 | 0 |
| Neutropenia | 1 | 2 | 0 | 2 | 0 |
| Anemia | 0 | 1 | 1 | 3 | 0 |
| Thrombocytopenia | 1 | 1 | 1 | 0 | 2 |
| Esophageal stenosis | 2 | 0 | 0 | 3 | 0 |
| Esophageal fistula | 5 | 0 | 0 | 0 | 0 |
| Mucositis | 1 | 0 | 4 | 0 | 0 |
| Dermatitis | 0 | 3 | 2 | 0 | 0 |
| Pneumonitis | 0 | 5 | 0 | 0 | 0 |
HNSCC, head and neck squamous cell carcinoma; ESCC, esophageal squamous cell carcinoma; NAT, neoadjuvant therapy.
Discussion
In this retrospective study, we provide a strategy of simultaneous CCRT with 2-step SIB technique for synchronous HNSCC and ESCC. cCR was observed in 4 patients after CCRT, and pCR was noted in all patients with surgery. There were no treatment-related deaths. Hematological toxicities and esophageal stenosis recovered after intensive care, jejunostomy and stenting.
Surgical resection has been the standard treatment for synchronous HNSCC and ESCC previously 3) (16-19). However, the invasiveness of operation may compromise quality of life by causing vocal and swallowing dysfunctions. As for treatment outcomes of synchronous HNSCC and ESCC, Morita et al. reviewed 38 patients with simultaneous definitive CCRT and 15 patients with simultaneous resection. The 3- and 5-year overall survival (OS) after definitive CCRT were 49% and 44%, and the 3- and 5-year OS after resection were both 67%. However, 3 patients (20%) developed postoperative complications, including anastomotic leakage in 2 patients and hypoxia in 1 patient (20). Park et al. reported that 2-year OS in patients with simultaneous definitive RT for synchronous HNSCC and ESCC was 68% (21). Chen et al. evaluated 60 patients with locally advanced synchronous HNSCC and ESCC. 1- and 2-year survival rates were 52% and 13% (2). These results indicated that simultaneous definitive CCRT for synchronous HNSCC and ESCC is an effective and safe treatment. Because esophageal cancer has a much poorer outcome than head and neck cancer, the prognosis would mainly depend on the stage and management of esophageal cancer (2,22,23). Radical resection of the esophageal cancer has been regarded most beneficial to improve survival by aids of neoadjuvant CCRT. Taken together, simultaneous CCRT to synchronous tumors with goal to control head and neck cancer and to radically resect esophageal cancer might be a practical strategy.
To accomplish this goal, the design of RT concerning both tumor control and normal tissue toxicity is of critically important. Moreover, the radiation tolerance consideration for potential site of anastomosis in neck is also mandatory to avoid leakage. In this study, RT related toxicities were well analyzed with dose constrained by Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC). The anastomosis of gastric tube and proximal esophagus was fixed to anterior cervical ligament (17,20). Therefore, we assessed the RT dose of lower neck carefully. The RT dose of lower neck especially supraclavicular fossa, which previously proposed to be covered by 56 Gy in 35 fractions as PTVL in HNSCC in our institution, was reduced to 43.2 Gy in 24 fractions, as PTVL in ESCC to prevent complications at anastomosis site. No recurrence or anastomotic leakage was noted over lower neck in our study.
For possibly greater hematological toxicities with larger RT field, the concurrent regimen of chemotherapy was weekly cisplatin (30 mg/m2) or carboplatin with weekly monitoring of hemogram. All patients tolerated well without reduction in planned dose of RT and chemotherapy.
There are several limitations in our study. It was retrospective with very small number of patients in one single institution. We report this preliminary experience by using this strategy of simultaneous CCRT with 2-step SIB technique for these two separate lesions with curative intent. This aggressive treatment was seemed to be related to good results in cCR and pCR, and it was well-tolerated by patients with acceptable complications. To accumulate more experience in using this treatment strategy, we established a flow chart to guide further clinical practice.
In conclusion, the treatment strategy using simultaneous CCRT with 2-step SIB technique for synchronous HNSCC and ESCC has the benefit of shortened treatment time and the possibility of inducing cCR. Esophagectomy is suggested after simultaneous CCRT for feasible patients to improve survival. Our strategy of simultaneous CCRT with 2-step SIB technique followed by evaluation for esophagectomy with curative intent is considered to be a safe and promising treatment option, which warrants further investigation in future clinical trials.
Acknowledgments
Funding: This study was supported by the MacKay Memorial Hospital [grant numbers MMH-E-107-13, MMH-E-106-13].
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tro.2018.07.08). YJC serves as an Editor-in-Chief of Therapeutic Radiology and Oncology. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board in our institution. Informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Canova C, Richiardi L, Merletti F, et al. Alcohol, tobacco and genetic susceptibility in relation to cancers of the upper aerodigestive tract in northern Italy. Tumori 2010;96:1-10. [Crossref] [PubMed]
- Chen YH, Lu HI, Chien CY, et al. Treatment Outcomes of Patients with Locally Advanced Synchronous Esophageal and Head/Neck Squamous Cell Carcinoma Receiving Curative Concurrent Chemoradiotherapy. Sci Rep 2017;7:41785. [Crossref] [PubMed]
- Fukuzawa K, Noguchi Y, Yoshikawa T, et al. High incidence of synchronous cancer of the oral cavity and the upper gastrointestinal tract. Cancer lett 1999;144:145-51. [Crossref] [PubMed]
- Wang WL, Wang CP, Wang HP, et al. The benefit of pretreatment esophageal screening with image-enhanced endoscopy on the survival of patients with hypopharyngeal cancer. Oral oncol 2013;49:808-13. [Crossref] [PubMed]
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). JAMA 1999;281:1623-7. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Orlandi E, Palazzi M, Pignoli E, et al. Radiobiological basis and clinical results of the simultaneous integrated boost (SIB) in intensity modulated radiotherapy (IMRT) for head and neck cancer: A review. Crit Rev Oncol Hematol 2010;73:111-25. [Crossref] [PubMed]
- Guillot T, Spielmann M, Kac J, et al. Neoadjuvant chemotherapy in multiple synchronous head and neck and esophagus squamous cell carcinomas. Laryngoscope 1992;102:311-9. [Crossref] [PubMed]
- Saeki H, Toh Y, Morita M, et al. The treatment outcomes of synchronous and metachronous esophageal squamous cell carcinoma and head and neck squamous cell carcinoma. Esophagus 2012;9:158-64. [Crossref]
- Wallach JB, Rosenstein MM, Kalnicki S. Localized synchronous squamous cell carcinomas of the esophagus and hypopharynx treated with definitive concurrent chemoradiotherapy with a unified radiotherapy plan. Curr Oncol 2014;21:e354-7. [Crossref] [PubMed]
- Shinoto M, Shioyama Y, Sasaki T, et al. Clinical results of definitive chemoradiotherapy for patients with synchronous head and neck squamous cell carcinoma and esophageal cancer. Am J Clin Oncol 2011;34:362-6. [Crossref] [PubMed]
- Welz S, Schmid A, Hehr T, et al. Treatment-outcome for synchronous head-and-neck and oesophageal squamous cell carcinoma. Radiother Oncol 2005;77:267-70. [Crossref] [PubMed]
- Dai KY, Huang WC, Leu YS, et al. Preliminary results of preoperative chemoradiation therapy for cervical esophageal squamous cell carcinoma with larynx preservation. Therapeut Radiol Oncol 2016;23:237-47.
- Rades D, Seidl D, Janssen S, et al. Comparison of weekly administration of cisplatin versus three courses of cisplatin 100 mg/m2 for definitive radiochemotherapy of locally advanced head-and-neck cancers. BMC Cancer 2016;16:437-44. [Crossref] [PubMed]
- Kang MH, Kang JH, Song HN, et al. Concurrent Chemoradiation with Low-Dose Weekly Cisplatin in Locally Advanced Stage IV Head and Neck Squamous Cell Carcinoma. Cancer Res Treat. 2015;47:441-7. [Crossref] [PubMed]
- Morita M, Kawano H, Otsu H, et al. Surgical resection for esophageal cancer synchronously or metachronously associated with head and neck cancer. Ann Surg Oncol 2013;20:2434-9. [Crossref] [PubMed]
- Morita M, Saeki H, Ito S, et al. Technical improvement of total pharyngo-laryngo-esophagectomy for esophageal cancer and head and neck cancer. Ann Surg Oncol 2014;21:1671-7. [Crossref] [PubMed]
- Tachimori Y, Watanabe H, Kato H, et al. Treatment for synchronous and metachronous carcinomas of the head and neck and esophagus. J Surg Oncol 1990;45:43-5. [Crossref] [PubMed]
- Matsumoto A, Watanabe M, Mine S, et al. Comparison of synchronous versus staged surgeries for patients with synchronous double cancers of the esophagus and head-and-neck. Dis Esophagus 2017;30:1-6. [PubMed]
- Morita M, Egashira A, Nakaji YU, et al. Treatment of Squamous Cell Carcinoma of the Esophagus Synchronously Associated with Head and Neck Cancer. In Vivo 2017;31:909-16. [PubMed]
- Park JW, Lee SW. Clinical outcomes of synchronous head and neck and esophageal cancer. Radiat Oncol J 2015;33:172-8. [Crossref] [PubMed]
- Elias D, Mamelle G, el Malt O, et al. Synchronous cancers of the esophagus and of the ORL area: results of combined treatments with esophagectomy. Bull Cancer 1991;78:173-8. [PubMed]
- Wind P, Roullet MH, Quinaux D, et al. Long-term results after esophagectomy for squamous cell carcinoma of the esophagus associated with head and neck cancer. Am J Surg 1999;178:251-5. [Crossref] [PubMed]
Cite this article as: Huang YM, Leu YS, Lee JC, Chen CH, Su NW, Lin HC, Huang WC, Chen YJ. Establishment of treatment strategy and preliminary outcome for synchronous head and neck and thoracic esophageal squamous cell carcinoma. Ther Radiol Oncol 2018;2:32.


