
Outcome of acute leukemia patients with central nervous system (CNS) involvement treated with total body or CNS irradiation before transplantation
Introduction
The central nervous system (CNS) is the most common site of extramedullary involvement in acute lymphoblastic leukemia (ALL) (1). CNS involvement (CNS+) at diagnosis of ALL is approximately 5–10%. CNS relapse occurs in approximately 7–15% of ALL patients who have received CNS prophylaxis. For acute myeloid leukemia (AML), CNS involvement is less common than ALL (2). There is prognostic impact of CNS involvement at diagnosis on long-term survival for ALL children according to some large international study group (3-5). As for adult ALL, the data regarding the prognostic impact of CNS involvement is less consistent. Results from MRC/ECOG E2993 did show inferior 5-year overall survival (OS) rate for ALL patients with CNS involvement compared with those without (29% vs. 38%, P=0.03) (1). AML patients with CNS involvement at diagnosis also had an inferior 5-year OS compared with patients without CNS involvement (11% vs. 30% at, P=0.004). ALL patients relapse with CNS involvement has very poor prognosis, with a median OS of 6 months. Site of relapse (with and without CNS involvement) was an independent prognostic factor for survival (6).
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) has been one of the post-remission treatment options for high-risk acute leukemia, with its anti-leukemic activity by graft-versus-leukemia effect. Patients with CNS involvement have possible poor prognosis and frequently undergo allo-SCT. However, there is no standard peri-transplantation approach to effectively reduce CNS relapse rate and improve survival after transplantation. Strategies used to improve outcome include total body irradiation (TBI) in conditioning regimen, CSI and cranial radiotherapy (RT), or intrathecal chemotherapy in peri-transplantation setting (7,8). The impact of intensified conditioning with TBI and CNS RT on outcome is still undetermined.
We included patients of ALL or AML, with history of CNS involvement before transplantation. The aim of this study was to investigate that whether TBI or CNS RT could improve outcomes for these high-risk patients. The investigated outcomes included relapse-free survival (RFS), CNS-RFS, and OS.
Methods
Patients
We retrospectively reviewed the database in NTUH SCT center from 1995 to 2016. The SCT database is collected retrospectively between 1995 to 2009 and prospectively after 2009. We included consecutive AML and ALL patients with CNS disease before SCT. CNS involvement was mandatory, either at initial presentation or relapse before transplantation. The definition of CNS involvement included one of these conditions: leukemic blasts in the cerebrospinal fluid, symptoms from cranial nerve palsy, or image-documented brain or spinal lesion related to leukemia. All patients underwent allo-HSCT. The conditioning for transplantation was mainly myeloablative, either with chemotherapy alone or chemotherapy in combination with TBI. With TBI, the chemotherapy regimens for conditioning were mainly composed of cyclophosphamide, cytarabine or etoposide. Without TBI, the myeloablative chemotherapy regimen was busulfan and cyclophosphamide. Few patients receiving chemotherapy conditioning with reduced intensity were also included.
Patients were classified into RT group and non-RT group. RT included TBI or CNS radiation, including cranio-spinal irradiation (CSI) or cranial RT. With cytogenetic changes recorded in the database, we risk-stratified cytogenetics according to European LeukemiaNet 2017 classification (9).
RT technique
During 1995 to March 2007, RT was delivered using cobalt-60 teletherapy. For TBI, parallel opposed anteroposterior fields were used with lung blocks to lower the lung dose. Since March 2007, linear accelerators have become predominant. A bilateral TBI technique has been developed that used rice-bag compensators as intensity modulators (IM-TBI). TBI was delivered with a hyperfractionation schedule (9–12 Gy, <1.8 Gy per fraction). CSI was performed using 2D technique consisting of bilateral opposed and posterior-anterior fields with moving junctions, to a dose of 18 Gy in 10 fractions, followed by cranial content boost 6 Gy in 3 fractions using 3D technique. A cumulative dose at cranial content was 24 Gy. For patients receiving cranial RT only, the range of prescribed dose was 20–25 Gy with 3D technique.
Statistical analysis
Patient characteristic variables between the groups with and without RT was assessed using Fisher’s exact test for categorical data and t-test for continuous data. We evaluated RFS, CNS-RFS, and OS for patients in the two groups. OS was defined as the period from the date of diagnosis to death after allo-HSCT. RFS was defined as the period from date of diagnosis to relapse or death after allo-HSCT. For CNS-RFS, time was measured from the date of allo-HSCT instead of the date of diagnosis, in order to evaluate the impact of pre-transplantation RT on CNS relapse after transplantation. Patients were censored at the last date of follow-up. Survival were calculated on the basis of Kaplan-Meier estimates, and log-rank test was used for assessing difference between Kaplan-Meier survival curves. Univariate Cox regression analysis was used optionally to further evaluate the OS difference. Cox proportional hazard regression model was carried out for multivariate analysis with variables of interest. All tests were two-tailed, and the significance level was set at P=0.05.
Results
Patient characteristics
Totally, there were 373 acute leukemia patients with allo-HSCT and among them, 55 (14.7%) patients with CNS involvement were included in this study. Within this cohort, 37 (67%) patients received TBI as conditioning regimens or CNS RT. The patient characteristics was demonstrated in Table 1. There were more ALL patients in RT group compared with the no-RT group (P=0.001). For CNS disease status, 43% of the RT group had CNS disease at relapse, compared with 28% at relapse of the non-RT group.
Table 1
| Characteristics | RT (n=37) | No RT (n=18) | P value |
|---|---|---|---|
| Age | |||
| Median (range) (years) | 17.2 (2.8–53.8) | 32 (14.2–67.4) | <0.001 |
| <18 (n) | 19 | 3 | 0.019 |
| ≥18 (n) | 18 | 15 | |
| Sex, n (%) | 0.563 | ||
| Female | 13 (35.1) | 10 (55.6) | |
| Male | 24 (64.9) | 8 (44.4) | |
| Leukemia type, n (%) | 0.001 | ||
| AML | 9 (24.3) | 13 (72.2) | |
| ALL | 28 (75.7) | 5 (27.8) | |
| Cytogenetics, n (%) | 0.565 | ||
| Good-intermediate | 21 (56.8) | 12 (66.7) | |
| Poor | 16 (43.2) | 6 (33.3) | |
| CNS, n (%) | 0.377 | ||
| Initial | 21 (56.8) | 13 (72.2) | |
| Relapse before SCT | 16 (43.2) | 5 (27.8) | |
| Radiation, n (%) | – | ||
| TBI | 30 (81.1) | – | |
| CSI | 2 (5.4) | – | |
| Cranial | 5 (13.5) | – | |
| Conditioning, n (%) | – | ||
| Myeloablative | 35† (94.6) | 14 (77.8) | |
| Reduced | 2‡ (5.4) | 4 (22.2) |
†, for the 35 patients, 30 of them received TBI in combination with cyclophosphamide, cytarabine or etoposide. The remaining five patients received intense chemotherapy (busulfan and cyclophosphamide) without TBI; ‡, the 2 patients had cranial RT only, and received reduced-intensity conditioning with busulfan, fludarabine, cyclophosphamide and ATG. RT, radiation therapy; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CNS, central nervous system; TBI, total body irradiation; CSI, cranio-spinal irradiation; ATG, antithymocyte globulin.
In RT group, most of the patients (81%) received TBI. The remaining patients received CSI or cranial RT only. In non-RT group, most of them (78%) received myeloablative conditioning. The remaining four patients received chemotherapy with reduced intensity.
Survival outcome
At a median follow-up of 27.7 months (range, 1.0–237.3 months), the 2-year OS was 72.5% for patients with RT, and 72.2% without RT (log-rank test, P=0.111). A total of 19 of 37 (51.3%) patients in RT group and 11 of 18 (61.1%) patients in non-RT group experienced relapse. The 2-year RFS was 69.4% for patients with RT, and 44.4% without RT (P=0.164). Eight of 37 patients (21.6%) in RT group had CNS relapse after allo-HSCT, compared with 5 of 18 patients (27.8%) in non-RT group. The 2-year post-transplantation CNS-RFS was 73.3% for patients with RT, and 74.1% without RT (P=0.742). As shown in Figures 1-3, there is no significant difference in OS, RFS, and CNS-RFS between patients in RT and non-RT groups. The difference of OS was also not statistically significant using univariate Cox regression analysis (P=0.116).
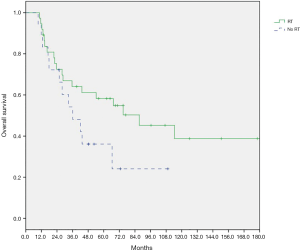
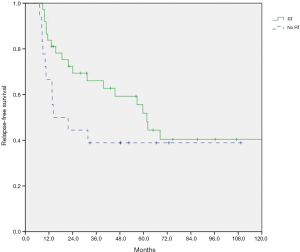
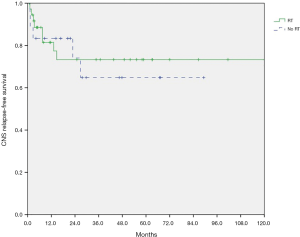
Subgroup analysis
The RT impact for AML and ALL patients were evaluated separately. For AML patients, the 2-year OS was 55.6% for patients with RT, and 69.2% without RT (P=0.286). For ALL patients, the 2-year OS was 78.1% for patients with RT, and 80% without RT (P=0.911). There was no significant difference of OS with or without RT for AML or ALL patients, respectively.
Relapse pattern
The relapse patterns of patients with and without RT were shown in Figure 4. Nineteen patients in RT group and 11 patients in no-RT group suffered from relapse after allo-HSCT. Bone marrow relapse predominated in both groups. For patients with RT, there seemed to be less extramedullary relapse.
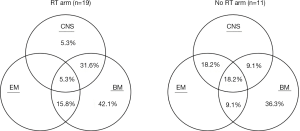
Prognostic factors
We used Log rank test (and univariate Cox regression model for age) to compare the difference of survival to identify prognostic factors. Factors analyzed included age at diagnosis, gender, different leukemia types (AML or ALL), cytogenetics risk-stratification (unfavorable-risk or others), initial CNS involvement or not, initial response to chemotherapy, relapse after initial chemotherapy and before transplantation, pre-transplantation disease status, CNS relapse or not, regimen intensity, and RT or non-RT. For OS, pre-transplantation disease status was significantly associated with outcome (P=0.008). For RFS, unfavorable-risk cytogenetics was the only significant poor prognostic factor (P=0.04). For CNS RFS, pre-transplantation CNS relapse was the only significant poor prognostic factor (P=0.011).
Cox regression univariate and multivariate analysis including variables mentioned above was performed. Pre-transplantation disease status (with complete response, P=0.03) and TBI or CNS RT (P=0.04) were significantly associated with better OS rate, as demonstrated in Table 2). Unfavorable cytogenetics had marginally significant effect on OS (P=0.07). For RFS, multivariate analysis revealed that the unfavorable-risk cytogenetics (P=0.02) and TBI or CNS RT (P=0.03) were the significant prognostic factors, as demonstrated in Table 3. For CNS RFS, regimen intensity was the only significant prognostic factor after multivariate analysis (P=0.04). TBI or CNS RT had marginally significant impact on CNS RFS (P=0.068).
Table 2
| Variables | Overall survival | ||||
|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | ||||
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age at diagnosis | 1.01 (0.99–1.03) | 0.55 | 0.98 (0.95–1.02) | 0.41 | |
| Gender | |||||
| Male | 1 | – | 1 | – | |
| Female | 1.14 (0.56–2.34) | 0.72 | 1.34 (0.61–3.01) | 0.45 | |
| Leukemia type | |||||
| AML | 1 | – | 1 | – | |
| ALL | 0.63 (0.31–1.29) | 0.21 | 1.09 (0.40–3.01) | 0.86 | |
| Cytogenetics | |||||
| Favorable | 1 | – | 1 | – | |
| Unfavorable | 1.33 (0.65–2.74) | 0.43 | 2.32 (0.93–5.77) | 0.07 | |
| Initial CNS | |||||
| Not involved | 1 | – | 1 | – | |
| Involved | 0.94 (0.45–1.97) | 0.87 | 1.86 (0.54–6.46) | 0.33 | |
| Initial response | |||||
| CR | 1 | – | 1 | – | |
| Non-CR | 1.28 (0.63–2.62) | 0.49 | 0.99 (0.38–2.60) | 0.98 | |
| Relapse after chemotherapy before transplantation | |||||
| Non-relapse | 1 | – | 1 | – | |
| Relapse | 1.54 (0.66–3.58) | 0.31 | 0.72 (0.23–2.26) | 0.57 | |
| Pre-transplantation CNS | |||||
| Not involved | 1 | – | 1 | – | |
| Involved | 1.47 (0.72–3.00) | 0.29 | 2.89 (0.75–11.22) | 0.13 | |
| Pre-transplantation status | |||||
| CR | 1 | – | 1 | – | |
| Non-CR | 2.62 (1.28–5.34) | 0.008 | 3.81 (1.17–12.37) | 0.03 | |
| TBI or CNS RT | |||||
| No | 1 | – | 1 | – | |
| Yes | 0.55 (0.26–1.16) | 0.12 | 0.32 (0.11–0.95) | 0.04 | |
| Regimen intensity | |||||
| Reduced | 1 | – | 1 | – | |
| Myeloablative | 0.85 (0.29–2.45) | 0.76 | 0.92 (0.19–4.45) | 0.92 | |
HR, hazard ratios; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CNS, central nervous system; CR, complete response; TBI, total body irradiation; RT, radiation therapy.
Table 3
| Variables | Relapse-free survival | ||||
|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | ||||
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age at diagnosis | 1.01 (0.99–1.03) | 0.46 | 0.99 (0.96–1.02) | 0.48 | |
| Gender | |||||
| Male | 1 | – | 1 | – | |
| Female | 1.03 (0.49–2.14) | 0.94 | 1.68 (0.71–4.00) | 0.24 | |
| Leukemia type | |||||
| AML | 1 | – | 1 | – | |
| ALL | 0.99 (0.47–2.07) | 0.97 | 1.17 (0.42–3.26) | 0.76 | |
| Cytogenetics | |||||
| Favorable | 1 | – | 1 | – | |
| Unfavorable | 2.19 (1.05–4.57) | 0.04 | 3.03 (1.24–7.36) | 0.02 | |
| Initial CNS | |||||
| Not involved | 1 | – | 1 | – | |
| Involved | 0.71 (0.34–1.46) | 0.35 | 1.45 (0.41–5.07) | 0.56 | |
| Initial response | |||||
| CR | 1 | – | 1 | – | |
| Non-CR | 1.35 (0.66–2.76) | 0.42 | 2.08 (0.79–5.49) | 0.14 | |
| Relapse after chemotherapy before transplantation | |||||
| No relapse | 1 | – | 1 | – | |
| Relapse | 1.31 (0.56–3.05) | 0.53 | 0.76 (0.23–2.50) | 0.65 | |
| Pre-transplantation CNS | |||||
| Not involved | 1 | – | 1 | – | |
| Involved | 1.63 (0.79–3.36) | 0.19 | 4.09 (0.94–17.76) | 0.06 | |
| Pre-transplantation status | |||||
| CR | 1 | – | 1 | – | |
| Non-CR | 1.28 (0.61–2.72) | 0.51 | 1.11 (0.39–3.18) | 0.85 | |
| TBI or CNS RT | |||||
| No | 1 | – | 1 | – | |
| Yes | 0.59 (0.28–1.25) | 0.17 | 0.30 (0.10–0.91) | 0.03 | |
| Regimen intensity | |||||
| Reduced | 1 | – | 1 | – | |
| Myeloablative | 0.78 (0.27–2.25) | 0.65 | 0.48 (0.11–2.14) | 0.33 | |
AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CNS, central nervous system; CR, complete response; TBI, total body irradiation; RT, radiation therapy.
Discussion
Our study has shown that radiation, such as TBI for conditioning or pre-HSCT CNS RT, may have benefit to improve OS and RFS. However, the RT impact on CNS RFS was only marginally significant. Aldoss in a single-center retrospective analysis included ALL patients with history of CNS involvement and tried to identify an effective strategy in peri-transplantation setting in order to reduce CNS relapse rate after transplantation (7). The investigated strategies included conditioning with TBI, cranial RT or post-transplantation intrathecal chemotherapy. None of these strategies had impact on the CNS relapse rate or OS. The status of CNS involvement (at diagnosis or at relapse) did not affect CNS-RFS according to Aldoss. Our study demonstrated potential OS benefit by TBI or CNS RT, probably due to inclusion of different cohort from those in Aldoss’s study. Similarly, our study showed that the status of CNS involvement was not predictive of CNS RFS after multivariate analysis.
The benefit of CNS RT had been demonstrated for AML patients. Mayadev et al. included AML patients undergoing allo-HSCT, with and without CNS involvement (10). Most of CNS-positive patients received TBI conditioning, and some of them received cranial RT or CSI (CNS RT) additionally. CNS-positive patients had better survival outcome and improved CNS relapse rate if undergoing CNS RT, compared with CNS-positive patients without CNS RT. With CNS RT, the 1- and 5-year CNS relapse rate for CNS-positive patients improved from 37% to 32%, and 46% to 37%, respectively. Furthermore, with CNS RT, the OS and CNS relapse rate for CNS-positive patients was comparable to CNS-negative patients. Our study has shown OS benefit from RT, but the impact was not significant in subgroup analysis for AML patients, probably due to small patient number of AML.
Gao recently demonstrated the benefit of augmenting RT, with adding cranial boost to TBI conditioning for ALL patients undergoing allogenic transplantation (11). For CNS-positive patients, 2-year CNS relapse rate was 0 in patients receiving cranial boost and 21% in patients without cranial boost (P=0.03). None of the patients who received a cranial boost relapsed in the CNS. Cranial boost is the only significant factor affecting CNS relapse for CNS-positive patients by univariate analysis. The benefit from cranial boost did not translate into OS or RFS.
Our study is a retrospective study. The results of the study, including the subgroup analysis, were limited by small patient number. Furthermore, the results might be influenced by selection bias and varied by individual treatment decision. The characteristics of patients in RT and non-RT group were also imbalanced. More patients with ALL were in the RT group, which might mitigate the RT effect. It was worth noting that the two OS curves with and without RT in our study were quite separate despite that the difference did not reach statistical significance by log-rank test or univariate Cox regression. The impact of TBI or CNS RT on OS became significant after eliminating the effect from cofounding factors by multivariate analysis. Although there was RT benefit on OS and RFS, the influence on CNS RFS was only marginally significant. The possible explanation e is the insufficient dose to CNS by TBI or cranial irradiation. The more intensive CNS-directed RT may be beneficial to the patients with CNS involvement. For example, combination of TBI cranial irradiation, or CSI to achieve adequate dose to brain (24 Gy) and spine (18 Gy) as treatment to CNS leukemia patients may be an option to improve CNS relapse rate, and probably to further improve RFS and OS. We need protocol-based treatment strategy, more patient number and longer follow-up period to clearly demonstrate RT effect in the future.
Conclusions
Our study has shown that radiation, including TBI conditioning or pre-transplantation CNS RT, may have impact on improving survival after transplantation for high-risk acute leukemia patients. Different treatment strategies such as novel medication or intensifying radiation may be an option to be investigated in the future.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tro.2018.07.04). SHK serves as an Associate Editors-in-Chief of Therapeutic Radiology and Oncology from Oct 2017 to Sep 2019. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lazarus HM, Richards SM, Chopra R, et al. Central nervous system involvement in adult acute lymphoblastic leukemia at diagnosis: results from the international ALL trial MRC UKALL XII/ECOG E2993. Blood 2006;108:465-72. [Crossref] [PubMed]
- Alakel N, Stölzel F, Mohr B, et al. Symptomatic central nervous system involvement in adult patients with acute myeloid leukemia. Cancer Manag Res 2017;9:97-102. [Crossref] [PubMed]
- Möricke A, Zimmermann M, Reiter A, et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia 2010;24:265-84. [Crossref] [PubMed]
- Gaynon PS, Angiolillo AL, Carroll WL, et al. Long-term results of the children's cancer group studies for childhood acute lymphoblastic leukemia 1983-2002: a Children's Oncology Group Report. Leukemia 2010;24:285-97. [Crossref] [PubMed]
- Silverman LB, Stevenson KE, O'Brien JE, et al. Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985-2000). Leukemia 2010;24:320-34. [Crossref] [PubMed]
- Fielding AK, Richards SM, Chopra R, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood 2007;109:944-50. [Crossref] [PubMed]
- Aldoss I, Al Malki MM, Stiller T, et al. Implications and Management of Central Nervous System Involvement before Allogeneic Hematopoietic Cell Transplantation in Acute Lymphoblastic Leukemia. Biol Blood Marrow Transplant 2016;22:575-8. [Crossref] [PubMed]
- Hamdi A, Mawad R, Bassett R, et al. Central nervous system relapse in adults with acute lymphoblastic leukemia after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2014;20:1767-71. [Crossref] [PubMed]
- Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017;129:424-47. [Crossref] [PubMed]
- Mayadev JS, Douglas JG, Storer BE, et al. Impact of cranial irradiation added to intrathecal conditioning in hematopoietic cell transplantation in adult acute myeloid leukemia with central nervous system involvement. Int J Radiat Oncol Biol Phys 2011;80:193-8. [Crossref] [PubMed]
- Gao RW, Dusenbery KE, Cao Q, et al. Augmenting Total Body Irradiation with a Cranial Boost before Stem Cell Transplantation Protects Against Post-Transplant Central Nervous System Relapse in Acute Lymphoblastic Leukemia. Biol Blood Marrow Transplant 2018;24:501-6. [Crossref] [PubMed]
Cite this article as: Kuo WH, Chen YH, Tsai CH, Liao XW, Kuo SH, Tang JL. Outcome of acute leukemia patients with central nervous system (CNS) involvement treated with total body or CNS irradiation before transplantation. Ther Radiol Oncol 2018;2:29.


