Treatment outcomes for pediatric pineoblastoma: a single institute experience in Taiwan
Introduction
The pineal gland was first named by Galen (c.130–210AD) due to its nut-shaped appearance, similar to the pinecones of the stone spine (1,2). The pineal gland is a neuroendocrine organ about 7×6×3 mm3 in size, and is situated deep in the midbrain in the pineal recess of the third ventricle (3). The pineal gland produces melatonin, a nocturnal hormone with diverse physiological functions that helps regulate the sleep patterns (4). Because of its location, tumors of the pineal gland can compress the cerebral aqueduct and cause symptoms related to hydrocephalus and intracranial hypertension (5).
The World Health Organization (WHO) classification of central nervous system (CNS) tumor 2007 and 2016 recognizes four major subgroups of pineal-parenchymal tumors: pineocytoma (WHO grade I), pineal-parenchymal tumors of intermediate differentiation (WHO grades II and III), and pineoblastoma (WHO grade IV). Tumor manifestations are the consequence of compression of the brain stem and consist of nausea/vomiting, visual disturbances, headache, mental deterioration, and cranial nerve deficits such as loss of up-ward gaze (Parinaud syndrome) (6).
Generally, pineoblastomas are treated in a similar manner to high-risk medulloblastomas. Surgical intervention is recommended if the tumor is operable, and further adjuvant treatment may be considered based on pathological result and metastatic risk. However, pediatric pineoblastomas carry a poor prognosis because of a high relapse rate and tendency for metastasis throughout the craniospinal axis. Because of its rarity and high mortality rate, treatment recommendations are not well-established due to small numbers of patients and limited evidence. It remains unclear what influences overall survival and outcomes in patients with pineal tumors.
The aim of this study was to examine the clinical outcomes of pediatric pineoblastoma patients after multimodality treatment at a single medical center in Taiwan.
Methods
Clinical data collection
The medical records of children with pathologically proven pineoblastomas treated at Taipei Veterans General Hospital between 1991 and 2006 were retrospectively reviewed. A total of 11 patients were identified, and data collected included age, sex, operation date, diagnoses date, initial signs and symptoms, neuroimaging data [presence of hydrocephalus and tumor size diagnosed by computed tomography (CT) or magnetic resonance imaging (MRI)], pathological findings, treatment modalities (operation, radiotherapy, chemotherapy, or combination), treatment-related information (the extent of excision, radiotherapy dose and duration, chemotherapy regimen and frequency), failure patterns, recurrence date, follow-up date, death date, and toxicities.
The extent of excision was recorded as gross total resection (GTR), defined as no evidence of remaining tumor, and subtotal resection (STR), and defined as any amount of residual tumor. Pathological diagnosis was defined as the final report after tumor removal or stereotactic biopsy.
The study endpoints included progression free survival and overall survival. Tumor status was evaluated according to the Response Evaluation Criteria in Solid Tumor (RECIST) guidelines by experienced neuroradiologists. Any recurrence, such as local regional recurrence, persistence of tumor at the original site, or distance metastasis based on imaging or pathological findings, was considered an event in the calculation of progression-free survival. Death from any cause was used to define overall survival. Duration was calculated from the date of diagnosis to the date of an event. Late toxicities were assessed using the Common Terminology Criteria for Adverse Effects, version 4.0. Permission to perform this retrospective study was obtained from Taipei Veterans General Hospital Institutional Review Board.
Statistical methods
Survival curves were estimated with the Kaplan-Meier method, and patients without events were censored at the last follow-up date. Univariate Cox regression analysis was used to identify possible risk factors for overall survival. Factors examined included sex, age, hydrocephalus status, treatment modalities, and distant metastasis at diagnosis. All analyses were performed using R (version R-3.4.3; http://www.r-project.org) and SPSS (version 23.0, SPSS Inc., Chicago, USA). A two-sided P value <0.05 was considered statistically significant.
Results
Eleven patients with complete data of interest were included in the analysis (Table 1). A variety of treatment approaches were used for the 11 patients (Table 2). The median follow-up time was 12.4 months (10 years for patients who were still alive). There were 7 females (63.6%) and 4 males (36.4%), with a median age at diagnosis of 5.25 years (range, 1.47–16.8 years). There are 6 patients (54.5%) whose age is under 3 years old and 5 patients (45.5%) whose age is over 3 years old.
Table 1
| Characteristic | Number of patients (N=11) (%) |
|---|---|
| Age | |
| Median (range), years | 5.25 (1.47–16.8) |
| <3 years old | 6 (54.5) |
| ≥3 years old | 5 (45.5) |
| Sex | |
| Male | 4 (36.4) |
| Female | 7 (63.6) |
| Presenting symptoms | |
| Nausea/vomiting | 9 (81.8) |
| Headache | 4 (36.4) |
| Unstable gait | 2 (18.2) |
| Vision change | 2 (18.2) |
| Seizure | 2 (18.2) |
| Limbs weakness | 1 (9.1) |
| Conscious disturbance | 1 (9.1) |
| Initial disease status | |
| Localized disease | 6 (54.5) |
| Distant metastasis | 5 (45.5) |
| Hydrocephalus at diagnosis | 8 (72.7) |
| Initial treatment | |
| OP + RT + C/T | 3 (27.3) |
| OP + C/T | 3 (27.3) |
| RT + C/T | 2 (18.2) |
| C/T only | 3 (27.3) |
| Salvage treatment | |
| Surgery | 1 (9.1) |
| Radiation | 3 (27.3) |
| Chemotherapy | 3 (27.3) |
| Initial radiotherapy field | |
| Craniospinal + focal | 5† |
| Radiotherapy dose (median) | |
| Focal dose (Gy) | 54.7 |
| Craniospinal dose (Gy) | 33.1 |
| Last reported status | |
| Alive | 3 (27.3) |
| Dead | 8 (72.7) |
†, all patients (5 patients) who received radiotherapy as initial treatment received craniospinal irradiation with focal boost at cranial area. OP, operation; RT, radiotherapy; C/T, chemotherapy.
Table 2
| Pt | Age (year) | Sex | Mets at Dx | Surgical resection | RT CSI (Gy) | RT focal (Gy) | C/T | Recurrence | Failure pattern | Salvage Tx | Last status | Survival years |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6.3 | M | N | STR | 39.2 | 51.4 | Y | Y | Local | – | Died | 3.59 |
| 2 | 1.9 | F | N | STR | – | – | Y | Y | Mets | RT | Died | 2.27 |
| 3 | 2.8 | M | Y | – | – | – | Y | Y | Mets | C/T | Died | 0.65 |
| 4 | 16.8 | F | Y | GTR | 37.5 | 53.5 | Y | Y | Mets | OP + C/T | Died | 8.07 |
| 5 | 8.8 | M | N | – | 25.6 | 55.6 | Y | N | – | – | Alive | 15.87 |
| 6 | 1.5 | F | N | GTR | – | – | Y | Y | Mets | – | Died | 0.63 |
| 7 | 2.2 | M | Y | – | – | – | Y | Y | Mets | – | Died | 0.91 |
| 8 | 1.7 | F | Y | – | – | – | Y | Y | Mets | RT | Died | 1.53 |
| 9 | 1.5 | F | N | STR | – | – | Y | Y | Mets | RT | Died | 2.10 |
| 10 | 7.2 | M | N | STR | 25.5 | 55.5 | Y | N | – | – | Alive | 11.64 |
| 11 | 7.0 | F | Y | – | 37.8 | 57.6 | Y | N | – | – | Alive | 11.12 |
Pt, patient; Mets at Dx, metastasis status at diagnosis; RT CSI, radiotherapy craniospinal dose; RT focal, radiotherapy focal dose; OP, operation; C/T, chemotherapy; salvage Tx, salvage treatment; M, male; F, female; N, no; Y, yes; STR, subtotal resection; GTR, gross total resection.
Nausea and vomiting (81.8%) were the most common initial presenting symptoms, and headache (36.4%), unstable gait (18.2%), eye movement disturbance (18.2%), seizures (18.2%), limbs weakness (9.1%), and consciousness disturbance (9.1%) were also reported. Five patients (45.5%) had distant metastasis at the time of diagnosis, and 8 patients (72.7%) had hydrocephalus at the time of diagnosis.
For the initial treatment, 3 patients (27.3%) underwent surgical resection plus post-operative radiotherapy and chemotherapy, 3 patients (27.3%) underwent surgical resection plus chemotherapy, 2 patients (18.2%) underwent radiotherapy plus chemotherapy, and 3 patients (27.3%) received chemotherapy only. Five patients received craniospinal irradiation (CSI) with focal tumor boost, and the median CSI dose was 33.1 Gy (range, 25.6–39.2 Gy), and the median focal dose was 54.7 Gy (range, 51.4–57.6 Gy).
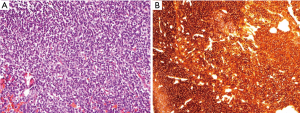
On pathological examination, the pineoblastomas were composed of densely packed small cells with round to oval-shaped nuclei and scanty cytoplasm, and occasional rosettes were sometimes identified. Representative hematoxylin and eosin (H&E) stained and synaptophysin stained images are shown in Figure 1.
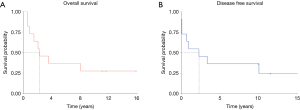
The median overall survival time for the entire cohort was 2.3 years (Figure 2), with 2- and 5-year survival rates of 63.6%, and 36.4%, respectively. At the end of follow-up, 8 patients (72.8%) died after serial treatment, and in 6 of them (75%) treatment failed because of extensive seeding.
Survival curves were estimated with the Kaplan-Meier method, and significant differences (P<0.05) were noted when with respect to age at diagnosis (over 3 years old or under 3 years old) (P=0.0014). (Figure 3). There was no difference in survival between males and females, with or without surgery, or whether or not metastases were present at the time of diagnosis. Although statistical significance was not reached, we noted males and those who did not have distant metastasis at diagnosis tended to have a better prognosis.
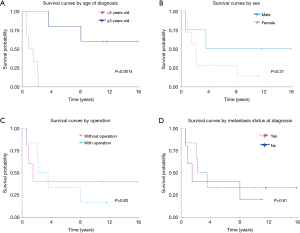
Univariate Cox regression was used to evaluate associations of initial treatment modalities, hydrocephalus at diagnosis, distant metastasis at diagnosis, sex, and age with survival and no significant associations were found (Table 3).
Table 3
| Factor | P value |
|---|---|
| Treatment | |
| OP + C/T | 0.944 |
| RT + C/T | 0.949 |
| C/T only | 0.939 |
| OP + RT + C/T | Reference |
| Hydrocephalus | |
| Yes | 0.063 |
| No | Reference |
| Distant metastasis at diagnosis | |
| Yes | 0.609 |
| No | Reference |
| Sex | |
| Female | 0.320 |
| Male | Reference |
| Age | |
| <3 years old | 0.160 |
| ≥3 years old | Reference |
OP, operation; RT, radiotherapy; C/T, chemotherapy.
At the final follow-up, 3 patients (27.2%) were alive without disease recurrence. Among the living patients, one developed hypogonadotropic syndrome with short status at the age of 7, one experienced high pitch hearing lost at the age of 22, and the other patient did not develop any obvious complications during follow-up.
Discussion
The pineal gland produced melatonin which is regulated by the light and dark cycle (4). Tumors in the pineal region are rare, accounting for only 0.4–1.0% of intracranial tumors (7). The 2016 WHO Classification of Tumors of the CNS recognizes four main groups of tumors in the pineal region: pineocytoma, pineal parenchymal tumor of intermediate differentiation, papillary tumor of the pineal region, and pineoblastoma (8). Pineoblastoma is a WHO grade IV tumor of the CNS that features aggressive growth and early distant metastasis. Histopathologically, pineoblastomas are composed of small blue round cells with salt and pepper chromatin, inconspicuous nucleoli, and scant cytoplasm. Hypercellular tumors with uniform cells, high nuclear-to-cytoplasmic ratio, and occasional rosettes are also easily noted features (9).
Pineoblastomas are a rare malignant tumor, with an estimated incidence of <0.1% of all intracranial tumors, making the development of treatment consensus difficult (10). The lesions are more common in children and adolescents than in adults, with an average age at diagnosis of 13 years. The survival rate of pediatric patients is markedly worse than adult patients (11). In our study, we reviewed the treatment outcomes of 11 patients with pineoblastomas treated at our hospital. The median overall survival was 2.3 years, and only three patients were long-term survivors after treatment. Our results confirmed the rarity, and poor prognosis of this malignant disease.
Due to its aggressive behavior, pineoblastomas are usually treated in a manner similar to high-risk medulloblastomas. Operation is the first choice of treatment, and maximal safe surgical resection is expected, although technically difficult due to the deep location of the pineal gland. Adjuvant radiotherapy with CSI and a local boost are usually given, and often combined with systemic chemotherapy to control or prevent distant metastasis (10). The current consensus of medulloblastoma grouping has moved from histologically to genetically defined subgroups as a result of better understanding of medulloblastoma biology (12). Four genetic (molecular) groups of medulloblastoma are now widely accepted: wingless (WNT)-activated, sonic hedgehog (SHH)-activated, and the numerically designated “group 3” and “group 4” (13). New risk stratifications and therapeutic combinations have been developed for medulloblastomas to individualize treatment approaches (12). Because of the limited patient numbers, there is no data available for developing molecular or genetic subtypes of pineoblastomas. Further studies are needed to give clinicians better prognostic prediction and treatment suggestions for pineoblastoma.
Several factors have been examined to predict the prognosis of pineoblastoma patients, including age, sex, distant metastasis at diagnosis, hydrocephalus at diagnosis, residual tumor after operation, and initial treatment modalities. Age at diagnosis is an important prognostic factor. Tate et al. reported that infant pineoblastomas demonstrated a 0% 1-year survival rate among children under 3 years old (14). Our study also found a significant difference in survival for children over and under 3 years of age. A study the summarized the existing literature of 299 pineoblastoma patients reported a 5-year survival rate of 15% for children younger than 5 years old, compared to 57% for children older than 5 years (14). Another study of 31 patients with pineoblastomas with a median age of 18.2 years showed a 5-year survival of 62.6% (10). A study that analyzed the data of 12 European centers reported a 5-year survival rate of 10% among 29 pineoblastoma patients with a median age of 12.5 years, and the median overall survival ranged from 1.3 to 2.1 years (15). In our study, the median overall survival for the entire cohort was 2.3 years, and the 5-year survival rate was 36.4%. With a median age at diagnosis of 5.25 years, our study consisted of mostly pediatric patients and associated poor survival rate.
Residual disease after operation is also an important prognostic factor for patients with pineoblastomas. One study reported a 5-fold increase in the independent proportional hazard of death for residual tumor after operation compared to gross total tumor removal (14). Interestingly, some studies have reported long-term survival for patients who received biopsy and radiation only. One recent paper from Iran reported a 22 years old patient with pineoblastoma and received biopsy and Gamma Knife radiosurgery over the cranial area with 14 Gy. The treatments have shown excellent tumor control without progression after 45 months of follow-up until the study ended (6). A case report in the Journal of Neurosurgery reported an unusually long survival of a pineoblastoma patient with a 9-year follow-up period after stereotaxic biopsy, a shunting procedure, and radiotherapy (16). Another case report showed successful combination chemotherapy using cisplatin, vinblastine, and bleomycin with small-dose irradiation (25 Gy) to treat a pineoblastoma patient with metastasis over the lumbar region through the cerebrospinal fluid, and suggested that combination treatment may be an option for disseminated pineoblastomas (17). Barlas et al. described the results of six patients treated with stereotactic biopsy, cerebrospinal fluid diversion, and fractionated radiotherapy (18). The overall survival rate was 80%±17.89% at 28 months, suggesting that non-surgical treatment is acceptable as an initial treatment. In our study, two of our three long-term survivors did not receive an operation, indicating that combine radiotherapy with chemotherapy may have the potential to achieve good treatment results. However, due to the limited number of patient the statistical power is not sufficient to draw any conclusions.
Distant metastasis at diagnosis is considered a poor prognostic factor, and has been discussed in prior studies. A Children’s Cancer Group (CCG-921) study found that 66% of patients with distant metastasis at diagnosis developed progressive disease within 32 months, as compared to 29% without distant metastasis at diagnosis (19). Worth mentioning, one study that reviewed 16 patients with pineoblastomas reported a disease-free survival of more than 11 years after diagnosis for one patient with distant metastasis (1). Similarly, in our study, one patient was diagnosed as having distant metastasis with tumor seeding over the posterior cranial fossa and thoracic spine, and her disease was successfully controlled with serial treatment. Survival curves estimated with the Kaplan-Meier method in our study did not show a significant difference between patient with and without distant metastasis at diagnosis. These results indicate that acceptable outcomes are possible for patients with distant metastasis at diagnosis.
CSI is important for disease control in patients with pineoblastomas. One recent study reported that recurrence was more likely in patients treated with focal irradiation only compared with patients treated with CSI (median dose: 36 Gy) with an initial focal boost (10). Another study of 34 pineoblastoma patients (age >16 years at diagnosis) from Japan reported that a threshold dose of >40 Gy over the cranial area significantly improved survival (20). Schild et al. retrospectively review of 15 patients with pineoblastomas, and found that patients who received >50 Gy over gross disease in the cranial area were 86% less likely to experience local recurrence (5). In our study, no patients were treated with a focal dose less than 50 Gy, and the median CSI dose was 33.1 Gy.
Our study has several limitations. Due to the limited number of patients, our study did not have the statistical power to determine significance by univariate Cox regression of the initial treatment type, age at diagnosis, sex, hydrocephalus at diagnosis, and distant metastasis at diagnosis. The radiotherapy dose and chemotherapy regimens varied, which make it difficult to provide definite treatment recommendations. Although proton therapy is a treatment trend in treating pediatric patients in developed countries (10), radiotherapy in our study was confined to photon therapy. We don’t have proton facilities in our hospital currently.
In summary, pediatric patients with pineoblastomas have a poor prognosis. In our study, survival was associated with age at diagnosis. Radiotherapy plus chemotherapy can provide long-term survival, and patients with distant metastasis at diagnosis may also achieve long-term survival with proper treatment.
Acknowledgments
Funding: This work was supported from grants from the Taipei Veterans General Hospital (No. V102B-008 and V105A-013), the Ministry of Science and Technology, Taiwan (NSC 102-2314-B-075-058), the Department of Health, Taiwan (No. DOH102-TD-C-111-007), and partial support from the Charity Foundation of JUT Land Development Group, Taiwan.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tro.2018.07.03). YML serves as an unpaid editorial board member of Therapeutic Radiology and Oncology from Oct 2017 to Sep 2019. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Permission to perform this retrospective study was obtained from Taipei Veterans General Hospital Institutional Review Board (No. 97-07-05A). Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gilheeney SW, Saad A, Chi S, et al. Outcome of pediatric pineoblastoma after surgery, radiation and chemotherapy. J Neurooncol 2008;89:89-95. [Crossref] [PubMed]
- Gaillard F, Jones J. Masses of the pineal region: clinical presentation and radiographic features. Postgrad Med J 2010;86:597-607. [Crossref] [PubMed]
- Borjigin J, Zhang LS, Calinescu AA. Circadian regulation of pineal gland rhythmicity. Mol Cell Endocrinol 2012;349:13-9. [Crossref] [PubMed]
- Macchi MM, Bruce JN. Human pineal physiology and functional significance of melatonin. Front Neuroendocrinol 2004;25:177-95. [Crossref] [PubMed]
- Schild SE, Scheithauer BW, Schomberg PJ, et al. Pineal parenchymal tumors. Clinical, pathologic, and therapeutic aspects. Cancer 1993;72:870-80. [Crossref] [PubMed]
- Motiei-Langroudi R, Sadeghian H, Soleimani MM, et al. Treatment Results for Pineal Region Tumors: Role of Stereotactic Biopsy Plus Adjuvant Therapy vs. Open Resection. Turk Neurosurg 2016;26:336-40. [PubMed]
- Dhall G, Khatua S, Finlay JL. Pineal region tumors in children. Curr Opin Neurol 2010;23:576-82. [Crossref] [PubMed]
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;131:803-20. [Crossref] [PubMed]
- Gener MA, Conger AR, Van Gompel J, et al. Clinical, Pathological, and Surgical Outcomes for Adult Pineoblastomas. World Neurosurg 2015;84:1816-24. [Crossref] [PubMed]
- Farnia B, Allen PK, Brown PD, et al. Clinical outcomes and patterns of failure in pineoblastoma: a 30-year, single-institution retrospective review. World Neurosurg 2014;82:1232-41. [Crossref] [PubMed]
- Tate MC, Rutkowski MJ, Parsa AT. Contemporary management of pineoblastoma. Neurosurg Clin N Am 2011;22:409-12. ix. [Crossref] [PubMed]
- Gopalakrishnan V, Tao RH, Dobson T, et al. Medulloblastoma development: tumor biology informs treatment decisions. CNS Oncol 2015;4:79-89. [Crossref] [PubMed]
- Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol 2012;123:465-72. [Crossref] [PubMed]
- Tate M, Sughrue ME, Rutkowski MJ, et al. The long-term postsurgical prognosis of patients with pineoblastoma. Cancer 2012;118:173-9. [Crossref] [PubMed]
- Fauchon F, Jouvet A, Paquis P, et al. Parenchymal pineal tumors: a clinicopathological study of 76 cases. Int J Radiat Oncol Biol Phys 2000;46:959-68. [Crossref] [PubMed]
- Uematsu Y, Itakura T, Hayashi S, et al. Pineoblastoma with an unusually long survival. Case report. J Neurosurg 1988;69:287-91. [Crossref] [PubMed]
- Kurisaka M, Arisawa M, Moriki A, et al. Successful combination chemotherapy (cisplatin, vinblastine, and bleomycin) with small-dose irradiation in the treatment of pineoblastoma metastasized into spinal cord: case report. Surg Neurol 1993;39:152-7. [Crossref] [PubMed]
- Barlas O, Bayindir C, Imer M, et al. Non-resective management of pineoblastoma. Minim Invasive Neurosurg 2000;43:163-70. [Crossref] [PubMed]
- Jakacki RI, Zeltzer PM, Boyett JM, et al. Survival and prognostic factors following radiation and/or chemotherapy for primitive neuroectodermal tumors of the pineal region in infants and children: a report of the Childrens Cancer Group. J Clin Oncol 1995;13:1377-83. [Crossref] [PubMed]
- Lee JY, Wakabayashi T, Yoshida J. Management and survival of pineoblastoma: an analysis of 34 adults from the brain tumor registry of Japan. Neurol Med Chir (Tokyo) 2005;45:132-41; discussion 141-2. [Crossref] [PubMed]
Cite this article as: Kang YM, Lin SC, Lee YY, Chang FC, Liang ML, Chen HH, Liu YM, Wong TT, Chen YW. Treatment outcomes for pediatric pineoblastoma: a single institute experience in Taiwan. Ther Radiol Oncol 2018;2:27.