
Single external oblique muscle metastasis of breast invasive lobular carcinoma: a case report
Introduction
Muscle metastases are very rare. The most common primary malignancies metastases to skeletal muscle are genital tumors (24.6%), followed by gastrointestinal tumors (21.3%) and urological tumors (16.4%) (1). It is extremely rare for breast cancer to metastasis to skeletal muscle. According to Surov et al. (2), metastases from breast cancer comprised only 3.3% of the 61 patients with muscle metastases. The most common metastatic sites were the extraocular muscles, followed by paraspinal muscles and gluteal muscles. Only two such cases of breast cancer metastasis to the ipsilateral abdominal muscle (3,4) and one case to the contralateral abdominal muscle (5) have been reported so far (Table 1). We present a rare case with abdominal external oblique muscle metastasis of right breast invasive lobular carcinoma.
Table 1
| Study | Metastatic site in skeletal muscle | Symptoms | Metastatic sites other than skeletal muscle | RT | OP | C/T |
|---|---|---|---|---|---|---|
| Right breast (Ogiya |
Right external oblique, internal oblique, transverse abdominal, and iliac muscles | Numbness; pain | Internal obturator lymph nodes | 50 Gy/25 fx | + | + |
| Right breast (Kim |
Right rectus abdominis muscle | Pain | Para-aortic lymph nodes | − | − | + |
| Left breast (Salemis |
Right rectus abdominis muscle | Pain | No | − | + | + |
| Right breast (our study) | Left external oblique muscle | No | T10 | 60 Gy/30 fx | + | + |
RT, radiotherapy; OP, operation; C/T, chemotherapy.
Case report
A 59-year-old female with history of right breast invasive lobular carcinoma [pT2N0M0, stage IIA (AJCC 5th staging)] received modified radical mastectomy with adjuvant chemotherapy and radiotherapy 50 Gy in 1999. Immunohistochemistry studies showed negative staining for estrogen receptor (ER) and progesterone receptor (PR), however, staining for human epidermal growth factor receptor 2 (HER2) was not performed. In 2002, she suffered from T10-spine metastasis and received 6-MV palliative photon radiotherapy 30 Gy in 10 fractions to T9–11 spine. From December 2003 to February 2005, she received pamidronate (Aredia) 90 mg monthly. In addition, Clodronate (Bonefos) 1,600 mg daily was prescribed from March 2005 to September 2007. In 2012, she had a left abdominal mass and received abdominal wall tumor resection. Pathological findings showed a 3.7 cm × 3.3 cm × 0.8 cm metaplastic invasive lobular carcinoma, and ER(+), PR(−), HER2(−) with positive surgical margin. The expression of ER showed a discordance between the primary breast cancer and the metastatic abdominal carcinoma. Then, she received hormone therapy with letrozole 2.5 mg per day after operation until second muscle metastasis was noted.
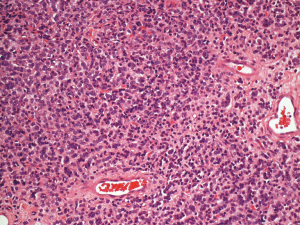
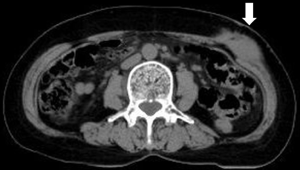
In 2016, she experienced a left abdominal painless mass at the same location underneath the previous surgical scar. Wide excision showed a 2.1 cm × 1.8 cm × 1.2 cm nodular tumor with positive deep-and-circumferential margin. The pathological findings of tumor resection revealed metastatic invasive lobular carcinoma again, while the immunohistological staining of tumor tissues revealed CK7(+), ER(+), PR(−), HER2(−), and E-cadherin(−) (Figure 1). Bone scan showed no obvious change in comparison with the previous examination. There were no lung metastatic lesions on chest X-ray and no abdominal organ metastasis on abdominal ultrasound. Mammography and breast ultrasound both showed Breast Imaging Reporting and Data System (BI-RADS) category 1. Carcinoembryonic antigen (CEA) and cancer antigen 15-3 (CA15-3) were checked every 3 months since 1999, and both examinations showed no abnormalities. Under the impression of right breast cancer with left external oblique muscle metastasis, rcT0N0M1, rpT0N0M1, stage IV (AJCC 7th staging), she received post-operative three-dimensional conformal radiotherapy 60 Gy in 30 fractions with image-guided. Computed tomography (CT) showed a left external oblique muscle 4.8 cm × 5.0 cm mass (Figure 2). The gross tumor volume (GTV) was defined as the gross extent of tumor as demonstrated by CT. The clinical target volume (CTV) was expanded as the GTV plus 1-cm margin. The planning target volume (PTV) contained a 0.3-cm expansion of CTV. The 6-MV photon beams were given through tangential opposed portals. The treatment plan was derived by using Pinnacle version 9.2. During radiotherapy, acute treatment-related sequelae including grade 1 radiation dermatitis was observed. She continued to receive chemotherapy and no evidence of abdominal recurrence.
Discussion
Skeletal muscle metastasis is very rare for breast cancer, with only 15 cases published in the literature. Among these 15 cases, only two cases of metastasis to the ipsilateral abdominal muscle (3,4) and one case to the contralateral abdominal muscle (5) have been reported so far. Because of the harsh environment, skeletal muscle is resistant to metastatic disease. These factors include muscle motion contributing to tumor destruction, harsh muscle pH and muscles’ ability to remove tumor-produced lactic acid, which would induce angiogenesis. Furthermore, natural killer cells and lymphocytes, which play an important role in the inhibition of tumor metastasis, may be active in skeletal muscle (6). Skeletal muscle metastasis is thought to be a sign of disseminated disease and is often associated with multiple organ metastases. In our case, other than external oblique muscle metastasis, only T10-spine was involved. The most common clinical presentation is a painful and firm mass. Our case had no symptoms in these two times.
The most common appearance of metastatic muscle lesions on unenhanced CT scans is an enlargement of a muscle, and under enhanced CT, it is illustrated as a rim-enhancing intramuscular mass with central hypo-attenuation. Magnetic resonance imaging (MRI) is the primary imaging technique for muscle disease. Metastatic muscle lesions showed low signal intensity on T1-weighted images, and high signal intensity on T2-weighted images (7). Many painless lesions may remain undiagnosed until death. Aftermath autopsy studies have shown the incidence of skeletal muscle metastases ranges from 0.03% to 17.5% (2). Differential diagnosis of skeletal muscle metastases are myopathy, sarcoma, hematoma, and intramuscular abscess. Pathologic diagnosis is very important because the treatment approach and disease prognosis will be quite different.
Several studies have shown a discordant ER, PR, and HER2 status between primary and metastatic sites in breast cancer. Liedtke et al. (8) reported that the incidence of discordance for ER, PR, and HER2 between metastatic and primary tumors was 18.4%, 40.3%, and 13.6% respectively. In our case, expression of ER showed discordance between the primary right breast cancer and the metastatic left abdominal tumor. Niikura et al. (9) pointed that their patients with discordant HER2 status had shorter survival than patients with concordant HER2 status (P=0.003). In our reported case, there were no primary site HER2 status records for diagnosis conducted in 1999 because the importance of HER2 was not emphasized at that time. At the time of publishing, no research has examined ER, PR, and HER2 status of the primary and metastatic skeletal muscle lesions. Immunohistochemical features of breast cancer associated with skeletal muscle metastasis are still unclear.
E-cadherin is a transmembrane glycoprotein that plays an essential role in mediating stability of cell adhesion. Li et al. (10) published a meta-analysis that suggested that decreased E-cadherin expression might be a poor prognosis factor and could be a potentially new gene therapy target for breast cancer patients. Our reported case also had decreased E-cadherin expression in muscle metastasis pathology.
Treatment for muscle metastasis is palliative, and no curative treatment is known until now. Treatment options include radiotherapy, surgery or chemotherapy. Surgery may be considered in selected cases with isolated painful masses. However, Surov et al. reported that in 11 post-operative cases, three cases had local recurrences (2); therefore, postoperative radiotherapy should be considered carefully. In our case, adjuvant radiotherapy must be considered due to positive margin in 2012. Radiotherapy is effective for decreasing lesion volume and reducing pain. Isolated recurrences (T10, 1st and 2nd muscle metastasis) were noted during 5-year interval of the treatment. Although the pathology did not have triple-negative characteristic, aggressive adjuvant therapy such as radiotherapy and chemotherapy should have been suggested.
In conclusion, we present a rare external oblique muscle metastasis of breast invasive lobular carcinoma case treated with surgery, chemotherapy and radiotherapy. Because muscle metastases are more common than we have expected, they should be included in differential diagnosis if patients with cancer history have an enlarging mass; even if such mass is not painful. Post-operative radiotherapy should have been taken into consideration due to high recurrence rate after surgery alone.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: CJH and MYH serve as an unpaid editorial board members of Therapeutic Radiology and Oncology from May 2020 to Apr 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tosios K, Vasilas A, Arsenopoulos A. Metastatic breast carcinoma of the masseter: case report. J Oral Maxillofac Surg 1992;50:304-6. [Crossref] [PubMed]
- Surov A, Hainz M, Holzhausen HJ, et al. Skeletal muscle metastases: primary tumours, prevalence, and radiological features. Eur Radiol 2010;20:649-58. [Crossref] [PubMed]
- Ogiya A, Takahashi K, Sato M, et al. Metastatic breast carcinoma of the abdominal wall muscle: a case report. Breast Cancer 2015;22:206-9. [Crossref] [PubMed]
- Kim YW, Seo KJ, Lee SL, et al. Skeletal muscle metastases from breast cancer: two case reports. J Breast Cancer 2013;16:117-21. [Crossref] [PubMed]
- Salemis NS. Skeletal muscle metastasis from breast cancer: management and literature review. Breast Dis 2015;35:37-40. [Crossref] [PubMed]
- Molina-Garrido MJ, Guillén-Ponce C. Muscle metastasis of carcinoma. Clin Transl Oncol 2011;13:98-101. [Crossref] [PubMed]
- Arpaci T, Ugurluer G, Akbas T, et al. Imaging of the skeletal muscle metastases. Eur Rev Med Pharmacol Sci 2012;16:2057-63. [PubMed]
- Liedtke C, Broglio K, Moulder S, et al. Prognostic impact of discordance between triple-receptor measurements in primary and recurrent breast cancer. Ann Oncol 2009;20:1953-8. [Crossref] [PubMed]
- Niikura N, Liu J, Hayashi N, et al. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J Clin Oncol 2012;30:593-9. [Crossref] [PubMed]
- Li Z, Yin S, Zhang L, et al. Prognostic value of reduced E-cadherin expression in breast cancer: a meta-analysis. Oncotarget 2017;8:16445-55. [PubMed]
Cite this article as: Hsu CJ, Hsu YC, Lu TY, Chen YT, Chai CY, Huang CJ, Huang MY. Single external oblique muscle metastasis of breast invasive lobular carcinoma: a case report. Ther Radiol Oncol 2018;2:22.


