
In vivo dosimetry of skin surface for breast cancer radiotherapy using intensity-modulated radiation therapy technique and helical tomotherapy
Introduction
Adjuvant radiotherapy (RT) has played an important role in multimodality treatment of breast cancer. For patients with early stage disease who receive breast conservative surgery (BCS), adjuvant RT to the residual breast tissue improved local control and survival (1). On the other hand, for patients with local advanced stage breast cancer who received mastectomy, adjuvant RT to chest wall and lymph node region reduced recurrent rate and breast cancer specific mortality (2,3).The target volume of RT after mastectomy typically includes ipsilateral chest wall, supraclavicular fossa (SCF), internal mammary lymph nodes (IMLN) and/or axillary lymph nodes (4,5).
Both linear accelerator-based intensity-modulated radiation therapy (IMRT) and helical tomotherapy (HT) are widely used for RT of breast cancer. Typical target volume of breast cancer is close to skin surface. However, most of treatment planning systems (TPS) in clinical practice do not provide accurate dose of skin surface and superficial target because inverse planning algorithm cannot accurately calculate the dose of build-up region (6-8). Skin surface will be potentially exposed to overdose or underdose due to inaccurate calculation in TPS, resulting in unexpected skin toxicity or decreased tumor control. The usual solutions used in clinical practice include adding bolus on the skin surface, using virtual bolus while treatment planning, and modified planning target volume (PTV) to avoid build-up region (9,10). On the other hand, some useful skills such as “skin flash” in IMRT and virtual bolus in HT are used to compensate the intra-fraction movement (11,12). However, accurate evaluation of skin dose becomes a challenge with the above approaches.
The purpose of this study is to measure the skin dose distributions of IMRT and HT by EBT films for patients who received breast cancer RT.
Methods
Eligibility criteria
Women who had undergone surgery, BCS or modified radical mastectomy (MRM), for invasive carcinoma or carcinoma in situ of the breast and were candidates for RT were included in this trial. Women who had breast reconstruction (either flap or any implantation), bilateral disease, wound infection before RT, surgical seroma requiring aspiration during treatment, or previous thoracic RT were excluded.
In this study, treatment machines were Synergy® (Elekta, Stockholm, Sweden) with Pinnacle planning system for linear accelerator-based IMRT, and TomoTherapy Hi-ART® (Accuray, Sunnyvale, CA, USA) for HT. Patients were assigned to IMRT or HT, depending on their decision. Daily image guided technique was essential to HT group with the use of megavoltage computed tomography (MVCT), but it was optional for IMRT group with the use of cone beam computed tomography (CBCT).
Target volume and treatment planning
The target volume was delineated according to Radiation Therapy Oncology Group (RTOG) atlas. Patients who underwent BCS were assigned to receive whole breast RT (WBRT). The clinical target volume (CTV) was ipsilateral residual breast tissue. The PTV was defined as CTV plus 5 mm margin and was modified to avoid the build-up region which was defined as 3 mm beneath the external surface. Prescribed dose was 50 Gy to PTV and/or a boost dose of 10 Gy to tumor bed. The daily fraction size was 2 Gy.
For patients who received post-mastectomy RT (PMRT) after MRM, the CTV included ipsilateral chest wall, axillary lymph node region, IMLN and SCF. The PTV was defined as CTV plus 5 mm margin. The PTV in PMRT group was also modified to avoid build-up region. Prescribed dose was 50 Gy to PTV and/or a boost dose of 10 Gy to surrounding region of operative scar. The daily fraction size was 2 Gy. All plans met the criteria of delivering more than 95% prescribed dose to 95% of the PTV and maximal dose less than 115%.
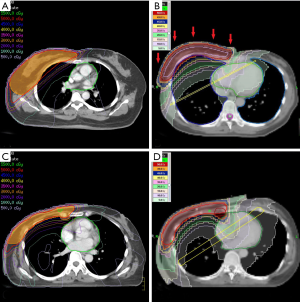
In WBRT group, an IMRT plan consisted of 2 to 7 IMRT field directions, depending on individual patient’s anatomy and PTV distribution shape (Figure 1A). In addition, “skin flash”, a field extension of 20 mm outside the skin surface, was used to deliver additional radiation on the space above the skin to cover the intra-fractional movement caused by respiration (Figure 1B). In HT plans for WBRT, a directional block at contralateral posterior thorax was designed to reduce the radiation from posterior and contralateral direction and reduce dose of lung and heart. Virtual bolus, which was a pretended material on the breast surface in treatment planning but absent during irradiation, was used in all the HT plans for WBRT to cover the patient’s movement. The density of virtual bolus in this study was defined as 1.0 g/cm3, and thickness was 10 mm.
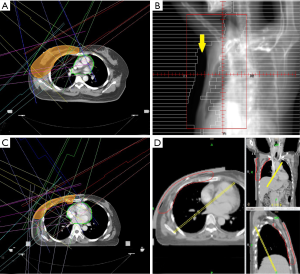
In PMRT group, an IMRT plan consisting of 7 IMRT field directions was designed with 20 mm skin flash (Figure 1C). A 5-mm tissue equivalent bolus was added on patient’s ipsilateral chest wall to ensure the superficial coverage. On the other hand, 10 mm tissue equivalent bolus was added for patient in PMRT group treated with HT. A directional block was also used to restrict the beam angle (Figure 1D).
Measurement of surface dose
For each patient included, the skin surface of the breast or chest wall was divided into six areas including upper-lateral area, lower-lateral area, upper-central area, lower-central area, upper-medial area and lower-medial area, which were coded as No.1, 2, 3, 4, 5, 6, respectively (Figure 2A,B).
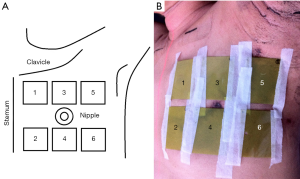
The “upper” and “lower” were defined as skin area above and below the horizontal level of the nipple; the “lateral”, “central” and “medial” were defined as skin area outside, along and inside the longitudinal level of the nipple, respectively. For patients who received mastectomy without nipple sparing procedure, the above definition of division was referred to contralateral nipple and ipsilateral midclavicular line.
GafChromic EBT3 (International Specialty Products, Wayne, NJ, USA) has been proven to a viable tool for megavoltage radiation dosimetry (13,14). The product was suitable to surface measurement near build-up region due to thin configuration (thickness of ~0.278 mm) and near-tissue equivalence (13). Each EBT3 film piece was cut into 4 cm × 5 cm. Six EBT3 film pieces were taped on the six skin areas of the breast or chest wall. If bolus material was used, the film pieces would be placed between the patient’s skin and bolus.
Each irradiated EBT3 film piece was scanned by Scanner Epson 10000XL at least 24 hours after irradiation. A region of interest (ROI) of 1 cm × 1 cm at the center was selected to collect the mean pixel value and standard deviation. Software FilmQA 2.20 was used for converting mean pixel value to absorbed dose of the skin areas. The calibration curve for pixel values of EBT3 film to absorbed dose was established on the same day when measurements were performed.
Absorbed dose of six EBT3 film pieces on six skin areas was defined as “film dose” of No.1, 2, 3, 4, 5 and 6, respectively. The average of six film dose in the same measurement was defined as “average surface dose”. The dose of medial region, central region and lateral region represented film dose of No.1 and 2, No.3 and 4, No.5 and 6, respectively. The dose of upper region contained film dose of No.1, 3 and 5; the dose of lower region contained film dose of No.2, 4 and 6.
For patient whom six EBT3 film pieces were unable to be placed on following above protocol, five-film-piece protocol was performed. The films No.1, 2, 5, 6, were placed under the same rules as six-film-piece protocol. Only one EBT film was placed at “central” region of ipsilateral breast or chest wall. The absorbed dose of the “central film” was included in calculation of average surface dose and was excluded while analysis of surface dose difference among skin regions.
Each patient received three times of EBT3 measurement in three consecutive fractions during the first phase of RT; none of measurement was performed in the phase of tumor bed boost.
To figure the dose difference between calculation by TPS and EBT3 measurement, point dose at skin surface on CT image was used to represent the surface dose calculated by TPS. In each plan, six points at the external surface contour were chosen from upper-lateral area, lower-lateral area, upper-central area, lower-central area, upper-medial area and lower-medial area, respectively, as the protocol of EBT3 film placement. If bolus material or virtual bolus was used, the point dose would be obtained from the interface between bolus and patient’s skin.
Statistic analysis
The average surface dose and film dose of No.1, 2, 3, 4, 5, and 6 from each skin area were compared between the different treatment techniques with the use of Mann-Whitney test. Furthermore, comparisons of medial surface dose (No.1 and 2), central surface dose (No.3 and 4) and lateral surface dose (No.5 and 6) were also performed using Friedman test. Comparisons of upper surface dose (No.1, 3 and 5) and central surface dose (No.2, 4 and 6) were also performed using Friedman test. This study was approved by the institutional review board as CGH P101029.
Results
Between July 2012 and December 2013, 30 patients were included; 17 patients were assigned to WBRT, 13 patients were assigned to PMRT. The characteristic of these patients was shown as Table 1.
Table 1
| Characteristics | Number (N=30) | Percent (%) |
|---|---|---|
| Median age at treatment (years) | 47.5 (34–72) | – |
| Treatment technique | ||
| IMRT | 17 | 56.7 |
| HT | 13 | 43.3 |
| Surgery | ||
| BCS | 17 | 56.7 |
| MRM | 13 | 43.3 |
| Side | ||
| Left | 10 | 33.3 |
| Right | 20 | 66.7 |
| Pathology stage | ||
| 0 | 2 | 6.7 |
| I | 13 | 43.3 |
| II | 3 | 10.0 |
| III | 10 | 33.3 |
| IV | 2 | 6.7 |
| T stage | ||
| 0 | 2 | 6.7 |
| T1 | 14 | 46.7 |
| T2 | 11 | 36.7 |
| T3 | 2 | 6.7 |
| T4 | 1 | 3.3 |
| N stage | ||
| N0 | 16 | 53.3 |
| N1 | 4 | 13.3 |
| N2 | 4 | 13.3 |
| N3 | 6 | 20.0 |
| M stage | ||
| M0 | 28 | 93.3 |
| M1 | 2 | 6.7 |
| Image-guided radiotherapy | ||
| Daily CBCT (Synergy) | 5 | 16.7 |
| Weekly CBCT (Synergy) | 12 | 40.0 |
| Daily MVCT (Hi-ART) | 13 | 43.3 |
IMRT, intensity-modulated radiation therapy; HT, helical tomotherapy; BCS, breast conserving surgery; MRM, modified radical mastectomy; CBCT, cone beam computed tomography; MVCT, megavoltage computed tomography.
In WBRT group, 7 patients received HT and 10 patients received linear accelerated-based IMRT; in PMRT group, 6 patients received HT and 7 patients received IMRT. The detail of treatment planning and dose distribution was shown in Table 2 and Figure 3. Each patient was irradiated by a daily fraction of 2 Gy and three times of skin dose measurement was performed in three consecutive fractions. Totally, 51 times of measurement were performed in WBRT group and 39 times of measurement were performed in PMRT group.
Table 2
| Treatment technique | WBRT (N=17) | PMRT (N=13) | |||
|---|---|---|---|---|---|
| IMRT (n=10) | HT (n=7) | IMRT (n=7) | HT (n=6) | ||
| Median number of IMRT fields | 6 | – | 7 | – | |
| Median PTV (mL)† | 770.39 (531.34–1389.71) | 918.78 (626.27–1012.22) | 756.58 (454.83–1677.25) | 734.47 (531.42–869.48) | |
| Median PTV coverage (%) | |||||
| V95%‡ | 99.19% (97.80–99.65%) | 99.89% (99.78–99.98%) | 98.72% (97.28–99.65%) | 99.70% (99.20–99.92%) | |
| V100%‡ | 94.24% (92.08–97.81%) | 97.71% (96.88–98.19%) | 96.33% (91.77–97.76%) | 97.09% (96.64–98.28%) | |
†, the PTV of nodal region was excluded; ‡, relative volume. WBRT, whole breast radiotherapy; PMRT, post-mastectomy radiotherapy; IMRT, intensity-modulated radiation therapy; HT, helical tomotherapy; PTV, planning target volume; V95%, PTV receiving ≥95% of the prescribed dose; V100%, PTV receiving ≥100% of the prescribed dose.
Six-film-piece protocol was unable to be placed properly for 3 patients. Two of them were assigned to WBRT group. One patient’s nipple located at relatively lower position so that No.4 EBT3 cannot be placed properly, and the other one has uneven surface of No.3 area. One patient in PMRT group presented with mildly erythematous change of operative scar at the No.3 area of the chest wall, and EBT film placement at that area was not recommended by physician. Five-film-piece protocol applied to these three patients.
WBRT group
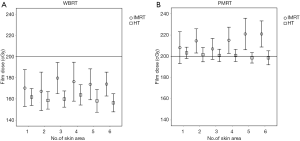
Table 3 and Figure 4A showed the result of EBT3 measurement and calculated dose by TPS in WBRT group. Patients received WBRT with HT received significantly lower film dose of No.1, 2, 3, 4, 5 and 6 than those with IMRT (P=0.001, 0.001, <0.001, 0.002, <0.001, <0.001, <0.001, respectively). HT also delivered significantly lower average surface dose than IMRT did with a difference of 6.88% of prescribed dose (IMRT versus HT: 86.57% versus 79.69%, P<0.0001). The calculated dose of IMRT in different skin regions was lower than film dose; while calculated dose of HT was higher than film dose in different skin regions.
Table 3
| Skin area | IMRT group (N=30) | HT group (N=21) | P value | |||
|---|---|---|---|---|---|---|
| Mean ± SD (cGy) | Percent (%)† | Mean ± SD (cGy) | Percent (%)† | |||
| No.1 | ||||||
| Film dose | 170.27±17.30 | 85.13 | 161.83±7.98 | 80.92 | 0.001 | |
| Calculated dose | 124.22±29.93 | – | 174.86±8.76 | – | ||
| No.2 | ||||||
| Film dose | 167.23±18.10 | 83.61 | 158.64±8.18 | 79.32 | 0.001 | |
| Calculated dose | 110.02±32.16 | – | 165.43±15.41 | – | ||
| No.3 | ||||||
| Film dose | 179.67±14.73 | 89.84 | 159.92±7.65 | 79.96 | <0.001 | |
| Calculated dose | 116.78±51.90 | – | 176.14±5.84 | – | ||
| No.4 | ||||||
| Film dose | 176.32±18.35 | 88.16 | 163.84±9.53 | 81.92 | 0.002 | |
| Calculated dose | 112.63±25.18 | – | 173.00±14.34 | – | ||
| No.5 | ||||||
| Film dose | 173.81±14.55 | 86.90 | 158.18±10.69 | 79.09 | <0.001 | |
| Calculated dose | 117.78±24.80 | – | 182.86±9.99 | – | ||
| No.6 | ||||||
| Film dose | 174.03±11.26 | 87.01 | 156.35±8.50 | 78.18 | <0.001 | |
| Calculated dose | 115.21±22.47 | – | 171.00±5.57 | – | ||
| Average surface dose | ||||||
| Film dose | 173.15±14.86 | 86.57 | 159.38±6.96 | 79.69 | <0.001 | |
| Calculated dose | 116.27±16.75 | – | 173.88±4.71 | – | ||
†, the percentage of dose data normalized to prescribed dose of 200 cGy per fraction. WBRT, whole breast radiotherapy; IMRT, intensity-modulated radiation therapy; HT, helical tomotherapy.
There was statistically significant dose difference among medial, central and lateral regions of patients received IMRT (84.42%, 89.00% and 87.34% of the prescribed dose, P<0.0001); IMRT delivered higher skin dose to the central regions than to the medial and lateral regions (Table 4). By contrast, HT resulted in relatively homogeneous dose distribution (mean dose of medial, central and lateral regions were 80.71%, 80.94% and 79.22%, P=0.199). As Table 4 showed, there was no statistically significant dose difference between upper and lower regions, whether delivered by IMRT (P=0.088) or HT (P=0.503).
Table 4
| Region | IMRT | HT | |||||
|---|---|---|---|---|---|---|---|
| Mean ± SD (cGy) | Percent (%)† | P value | Mean ± SD (cGy) | Percent (%)† | P value | ||
| Skin dose of medial region‡ | 168.84±18.44 | 84.42 | <0.001 | 161.41±7.93 | 80.71 | 0.199 | |
| Skin dose of central region‡ | 177.99±16.57 | 89.00 | 161.88±8.75 | 80.94 | |||
| Skin dose of lateral region‡ | 174.68±13.18 | 87.34 | 158.44±9.77 | 79.22 | |||
| Skin dose of upper region‡ | 174.41±15.90 | 87.21 | 0.088 | 159.98±8.92 | 79.99 | 0.503 | |
| Skin dose of lower region‡ | 172.39±16.44 | 86.20 | 159.39±9.10 | 79.70 | |||
†, data normalized to prescribed dose of 200 cGy per fraction; ‡, skin dose of medial region: No.1 and 2 film dose; skin dose of central region: No.3 and 4 film dose; skin dose of lateral region: No.5 and 6 film dose; skin dose of upper region: No.1, 3, 5 film dose; skin dose of lower region: No.2, 4, 6 film dose. WBRT, whole breast radiotherapy; IMRT, intensity-modulated radiation therapy; HT, helical tomotherapy.
PMRT group
The result of EBT3 measurement and calculated dose in PMRT group was shown in Table 5 and Figure 4B. The mean film doses delivered by IMRT and by HT were within 105–110% and 99–101% of the prescribed dose, respectively. Compared to the IMRT, the HT resulted in reduction in average surface dose of 7.06% (IMRT versus HT: 107.34% versus 100.28%, P<0.0001), which was much close to 100% of prescribed dose. Patients received PMRT with HT received significantly lower film dose of No.2, 3, 4, 5 and 6 than those with IMRT (P<0.001, 0.002, <0.001, <0.001, <0.001, respectively). Most of the calculated dose of IMRT in different skin regions was lower than film dose by measurement. Compared to measurement result, HT provided slightly higher calculated dose in skin surface.
Table 5
| Skin area | IMRT group (N=21) | HT group (N=18) | P value | |||
|---|---|---|---|---|---|---|
| Mean ± SD (cGy) | Percent (%)† | Mean ± SD (cGy) | Percent (%)† | |||
| No.1 | ||||||
| Film dose | 208.11±15.02 | 104.05 | 203.21±5.38 | 101.60 | 0.335 | |
| Calculated dose | 196.57±19.68 | – | 207.95±1.07 | – | ||
| No.2 | ||||||
| Film dose | 214.49±11.42 | 107.24 | 201.51±6.76 | 100.75 | <0.001 | |
| Calculated dose | 208.37±5.83 | – | 207.31±2.00 | – | ||
| No.3 | ||||||
| Film dose | 206.94±9.91 | 103.47 | 200.78±5.84 | 100.39 | 0.011 | |
| Calculated dose | 210.39±6.80 | – | 207.09±1.82 | – | ||
| No.4 | ||||||
| Film dose | 215.11±12.31 | 107.55 | 200.76±5.75 | 100.38 | <0.001 | |
| Calculated dose | 209.19±6.94 | – | 205.41±1.23 | – | ||
| No.5 | ||||||
| Film dose | 221.00±14.87 | 110.50 | 198.54±4.91 | 99.27 | <0.001 | |
| Calculated dose | 208.80±4.85 | – | 206.69±2.17 | – | ||
| No.6 | ||||||
| Film dose | 221.00±12.29 | 110.50 | 198.53±6.58 | 99.27 | <0.001 | |
| Calculated dose | 209.93±6.68 | – | 205.08±2.91 | – | ||
| Average surface dose | ||||||
| Film dose | 214.67±9.25 | 107.34 | 200.55±4.92 | 100.28 | <0.001 | |
| Calculated dose | 207.21±4.43 | – | 206.59±1.38 | – | ||
†, the percentage of dose data normalized to prescribed dose of 200 cGy per fraction. PMRT, post-mastectomy radiotherapy; IMRT, intensity-modulated radiation therapy; HT, helical tomotherapy.
Table 6 showed that IMRT delivered 5% higher dose to the lateral regions than medial and central regions (110.49%, 105.92% and 105.52% of prescribed dose, respectively, P=0.014); while lower skin regions received higher skin dose by IMRT than upper regions did with dose difference less than 3% (108.48% versus 106.14%, P=0.002).
Table 6
| Region | IMRT | HT | |||||
|---|---|---|---|---|---|---|---|
| Mean ± SD (cGy) | Percent (%)† | P value | Mean ± SD (cGy) | Percent (%)† | P value | ||
| Skin dose of medial region‡ | 211.83±13.96 | 105.92 | 0.014 | 202.36±6.08 | 101.18 | 0.005 | |
| Skin dose of central region‡ | 211.03±11.77 | 105.52 | 200.77±5.71 | 100.39 | |||
| Skin dose of lateral region‡ | 220.97±14.27 | 110.49 | 198.54±5.72 | 99.27 | |||
| Skin dose of upper region‡ | 212.27±14.89 | 106.14 | 0.002 | 200.84±5.63 | 79.99 | 0.313 | |
| Skin dose of lower region‡ | 216.95±12.17 | 108.48 | 200.26±6.38 | 79.70 | |||
†, data normalized to prescribed dose of 200 cGy per fraction. ‡, skin dose of medial region: No.1 and 2 film dose; skin dose of central region: No.3 and 4 film dose; skin dose of lateral region: No.5 and 6 film dose; skin dose of upper region: No.1, 3, 5 film dose; skin dose of lower region: No.2, 4, 6 film dose. PMRT, post-mastectomy radiotherapy; IMRT, intensity-modulated radiation therapy; HT, helical tomotherapy.
On the other hand, in PMRT group with HT, statistically inhomogeneous dose distribution was observed among medial, central, lateral regions, but the dose difference was less than 2% (101.18%, 100.39%, 99.27% of the prescribed dose, respectively; P=0.005).
Discussion
Surface dose of WBRT group
Almberg et al. presented results of a phantom study against surface dose of whole breast irradiation using different treatment planning techniques including conventional tangential fields, tangential IMRT and 7-field IMRT plan (15). The surface dose, measured by EBT film on the phantom surface, of conventional tangential fields was 45% to 65% of the prescribed dose. By contrast, the tangential IMRT and 7-field IMRT plan reduced surface dose by approximately 4–5% and 15–50%, respectively. Akino et al. also reported that IMRT presented reduction of surface dose compared to standard tangential beams (6). In our study, a median of 6 fields (range from 2 to 7) was used in IMRT plans for WBRT. The surface dose, measured by EBT film on the patient skin, of IMRT was 85–90% of the prescribed dose. Noted that mean surface dose in our study was relatively higher than dose in previous study. The possible reason might be the technique of “skin flash”, which delivered more fluence outside the PTV surface, to ensure the coverage of a moving target.
Zibold et al. reported the result of surface dose of WBRT using HT, measured by thermoluminescent dosimetry (TLD) on static phantom and patients (16). The mean dose on static phantom and patient was 80% of the prescribed dose. Although different dosimeter was used, in our study, similar result was presented that the surface dose of our patient received WBRT with HT was between 78% and 82% of the prescribed dose. The average surface dose delivered by tomotherapy was 7% lower than by IMRT in the WBRT group of prescribed dose, as shown in Table 3 and Figure 4A. The finding of our study was consistent with those of previous studies which showed that more fields with different angle of incidence reduced surface dose (6,15).
Quach et al., who used a hemicylindrical phantom to simulate chest skin and tangential photon beam, reported the maximal surface doses with 58% of prescribed dose was measured at the center with 90 degree of incidence angle, while the surface dose at beam entry position with zero degree of incidence angle was only 28% (17). From the other static phantom study for WBRT, the surface dose of central regions was approximately 20–40% higher than surface dose of medial and lateral regions; both tangential beam and 7-field IMRT provided in similar trend of surface dose distribution (15).
Our result of IMRT presented the same trend as the previous studies, but the difference of surface dose between central and other regions was only 4–5% (Table 4). It demonstrated that other factors, such as respiratory motion, set up error and skin flash technique, might eliminate the surface dose heterogeneity on real patient's skin. The relatively small difference might not be worthy to take into account in clinical practice. On the other hand, Tomotherapy delivered relatively homogeneous surface dose distribution in WBRT group with similar surface dose of central and bilateral skin regions (Table 4).
Panettieri et al. reported that the usual algorithms used for IMRT tend to underestimate the dose in the build-up region in comparison with Monte Carlo simulation (7). Ramsey et al. also revealed that the superficial calculated doses were overestimated by HT in comparison with measurement through a phantom study (8). Although the six positions where point dose was obtained in our study were not exactly equal to the positions of EBT3 films, a similar trend was observed in the result of WBRT group (Table 3).
Surface dose of PMRT group
Shiau et al. reported that four-field IMRT plans obtained more uniform surface dose distribution on static chest wall phantom than tangential wedged field plans did. The tangential wedged fields delivered higher surface dose to the central region than the bilateral border of PTV, with 75% and 51–56% of prescribed dose, respectively; while mean surface dose of four-field IMRT plan was 65% of prescribed dose, ranged from 54–70%, with a relatively uniform dose distribution (18). The dose heterogeneity caused by the oblique incidence, which was demonstrated by Quach et al, was reduced in the IMRT plans with multiple beam angles (17,18).
In our study, tissue-equivalent bolus was used for all patients in PMRT group. Our result indicated that adding bolus materials was effective to elevate skin dose up to 100% of prescription, as shown in Table 5 and Figure 4B. Both of IMRT and HT showed relatively homogeneous skin dose distribution, compared to the result of previous studies of tangential planning (6,15,17,18). The possible reason was that impact of incidence angle and build-up region might be mostly eliminated by the use of bolus materials. Additionally, the use of bolus materials might be also the reason of that calculated dose by TPS in PMRT group was closer to measurement than calculated dose in WBRT group. With 5–10 mm tissue equivalent bolus, skin surface would be away from the build-up region where inverse planning algorithm was unable to provide accurate dosimetry (6-8).
Noted that the lateral skin region received 4–5% higher skin dose than the other skin regions did in the PMRT group with IMRT. HT plans also delivered statistically inhomogeneous skin dose distribution in PMRT group, but the dose difference was less than 2%. Although the quality of dosimetry was strictly controlled through establishing calibration curve of EBT3 films on the same day when measurement was performed, there were other factors which had an impact on dose measurement such as respiratory movement, set up error, daily output, and homogeneity of beam profile. The relatively small dose difference might not be clinically significant.
Skin flash, virtual bolus and bolus
Skin flash technique and virtual bolus were widely used in breast RT to overcome the dose uncertainty caused by respiratory motion (9-12). Sankar et al. reported that 20 mm “skin flash” and 20 mm virtual bolus had similar effect to elevate surface dose and reduce hot spot in IMRT (11). In our result, dose difference about 7% of prescription was observed between 20 mm “skin flash” and 10 mm virtual bolus, despite different planning technique.
In our result of WBRT group, the surface dose did not reach the prescribed dose although skin flash or virtual bolus was used. If skin was defined as high risk area, a better solution to elevate superficial target coverage was covering skin by bolus materials. According to static phantom study, the build-up region for PMRT using 4 fields IMRT on chest wall is at least 2 mm beneath the surface (18). In our study for PMRT, tissue equivalent bolus with 5 mm in thickening and 20 mm skin flash were used in IMRT plans and mean surface dose was 7% higher than prescribed dose.
Kinoshita et al. showed that the average intra-fractional motion of the breast is 2.6±1.4 mm (mean ± standard deviation) for vertical direction (19). Considering lack of “skin flash” option in TPS of HT, 10 mm bolus was used for HT plans with more 5 mm in thickening than bolus used in IMRT to compensate the potential intra-fractional motion. In our result of PMRT group, the surface dose about 100% of prescription was obtained using this method.
Tournel et al. reported a static phantom study of Tomotherapy to simulate volume reduction with 10 mm bolus material added onto the phantom in the planning phase (20). Surface dose measurement was performed with bolus and repeated without bolus. A 19% drop in surface dose was observed in 10 mm reduction (20). Our result of PMRT group and WBRT group showed that skin surface dose of HT with 10 mm bolus and without bolus was about 100% and 80%, respectively. The efficacy of 10 mm bolus was similar to the result of Tournel et al.
Conclusions
Compared to the previous phantom studies, this study provided in vivo skin dose dosimetry in clinical situation with potential set up error and intra-fractional respiratory movement of real patients. The major limitation of this study was relatively small sample size and non-randomized design. Besides, the IMRT with Synergy was covered by national health insurance in Taiwan but the Tomotherapy was a self-paid treatment. It might contain bias of patient selection.
With the use of skin flash, virtual bolus or bolus materials, both IMRT and HT provided homogeneous surface dose distribution on the patient received breast RT, compared to result from static phantom studies.
In WBRT group, HT with virtual bolus led to more homogeneous dose distribution and nearing 7% lower surface dose than IMRT with skin flash did. For PMRT, despite lack of skin flash in TPS, HT plus 10 mm bolus still produced 100% of the prescribed dose to the skin surface. HT should be considered to be an option of breast RT and further investigation of correlation between adverse effect of the skin and surface dose distribution would be needed.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tro.2017.11.01). CJW serves as an unpaid editorial board member of Therapeutic Radiology and Oncology from Oct 2017 to Sep 2019. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the institutional review board as CGH P101029. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707-16. [Crossref] [PubMed]
- Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst 2005;97:116-26. [Crossref] [PubMed]
- McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014;383:2127-35. [Crossref] [PubMed]
- Poortmans PM, Collette S, Kirkove C, et al. Internal Mammary and Medial Supraclavicular Irradiation in Breast Cancer. N Engl J Med 2015;373:317-27. [Crossref] [PubMed]
- Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol 2014;15:1303-10. [Crossref] [PubMed]
- Akino Y, Das IJ, Bartlett GK, et al. Evaluation of superficial dosimetry between treatment planning system and measurement for several breast cancer treatment techniques. Med Phys 2013;40:011714 [Crossref] [PubMed]
- Panettieri V, Barsoum P, Westermark M, et al. AAA and PBC calculation accuracy in the surface build-up region in tangential beam treatments. Phantom and breast case study with the Monte Carlo code PENELOPE. Radiother Oncol 2009;93:94-101. [Crossref] [PubMed]
- Ramsey CR, Seibert RM, Robison B, et al. Helical tomotherapy superficial dose measurements. Med Phys 2007;34:3286-93. [Crossref] [PubMed]
- Thomas SJ, Hoole AC. The effect of optimization on surface dose in intensity modulated radiotherapy (IMRT). Phys Med Biol 2004;49:4919-28. [Crossref] [PubMed]
- Ashburner MJ, Tudor S. The optimization of superficial planning target volumes (PTVs) with helical tomotherapy. J Appl Clin Med Phys 2014;15:4560. [Crossref] [PubMed]
- Sankar A, Velmurugan J. Different intensity extension methods and their impact on entrance dose in breast radiotherapy: A study. J Med Phys 2009;34:200-5. [Crossref] [PubMed]
- Moliner G, Izar F, Ferrand R, et al. Virtual bolus for total body irradiation treated with helical tomotherapy. J Appl Clin Med Phys 2015;16:164-76. [Crossref] [PubMed]
- Casanova Borca V, Pasquino M, Russo G, et al. Dosimetric characterization and use of GAFCHROMIC EBT3 film for IMRT dose verification. J Appl Clin Med Phys 2013;14:4111. [PubMed]
- Chiu-Tsao S, Massillon-Jl G, Domingo-Muñoz I, et al. SU-E-T-96: Energy Dependence of the New GafChromic- EBT3 Film's Dose Response-Curve. Med Phys 2012;39:3724. [Crossref] [PubMed]
- Almberg SS, Lindmo T, Frengen J. Superficial doses in breast cancer radiotherapy using conventional and IMRT techniques: a film-based phantom study. Radiother Oncol 2011;100:259-64. [Crossref] [PubMed]
- Zibold F, Sterzing F, Sroka-Perez G, et al. Surface dose in the treatment of breast cancer with helical tomotherapy. Strahlenther Onkol 2009;185:574-81. [Crossref] [PubMed]
- Quach KY, Morales J, Butson MJ, et al. Measurement of radiotherapy x-ray skin dose on a chest wall phantom. Med Phys 2000;27:1676-80. [Crossref] [PubMed]
- Shiau AC, Chiu MC, Chen TH, et al. Surface and superficial dose dosimetric verification for postmastectomy radiotherapy. Med Dosim 2012;37:417-24. [Crossref] [PubMed]
- Kinoshita R, Shimizu S, Taguchi H, et al. Three-dimensional intrafractional motion of breast during tangential breast irradiation monitored with high-sampling frequency using a real-time tumor-tracking radiotherapy system. Int J Radiat Oncol Biol Phys 2008;70:931-4. [Crossref] [PubMed]
- Tournel K, Verellen D, Duchateau M, et al. An assessment of the use of skin flashes in helical tomotherapy using phantom and in-vivo dosimetry. Radiother Oncol 2007;84:34-9. [Crossref] [PubMed]
Cite this article as: Sung SY, Lee HY, Tu PC, Lin CH, Yu PC, Lui LT, Shaw S, Wu CJ, Nien HH. In vivo dosimetry of skin surface for breast cancer radiotherapy using intensity-modulated radiation therapy technique and helical tomotherapy. Ther Radiol Oncol 2017;1:2.


